Introduction
Prostate cancer remains the most commonly diagnosed noncutaneous malignancy among American men.1 Furthermore, radical prostatectomy is a mainstay of treatment for men with localized or locoregional disease. Indeed, 93.2% of men diagnosed with prostate cancer will receive treatment for their prostate cancer, and nearly 50% of such men will undergo surgery.2 Even after definitive local therapy, 32-35% of all men undergoing radical prostatectomy will experience biochemical recurrence (BCR) over a ten-year period.3-4 Despite the well-documented stage migration favoring earlier stage disease in the PSA era, patients with high-risk pathology after prostatectomy continue to pose a therapeutic challenge to treating urologists. Numerous studies have identified clinicopathologic features associated with recurrence following radical prostatectomy, including pre-operative PSA, pathologic stage, extraprostatic extension (EPE), seminal vesical invasion (SVI), positive lymph nodes and positive surgical margins (PSM).5-13 Nonetheless, the risk of BCR remains highly variable, with a contemporary series reporting 3-year BCR-free survival rates from 42.3-83.9%.9
Fortunately, the majority of patients with high-risk pathology do achieve an undetectable PSA nadir5, forcing clinicians to carefully weigh the possible benefits and harms associated with the delivery of adjuvant radiotherapy. Three prospective randomized-controlled trials have shown adjuvant radiotherapy (aRT) to improve BCR-free, metastasis-free and overall survival in patients with adverse pathologic features.14-16 However, because, at most, only 50% of these patients will ever exhibit BCR, there is a considerable risk of overtreatment.17 To this end, we sought to evaluate risk factors for BCR in a high-risk yet heterogenous cohort of patients that might reasonably be considered candidates for aRT.
Materials and Methods
After receiving Institutional Review Board (IRB) approval, we performed a retrospective cohort study to determine patterns of recurrence and patient-level predictors of BCR among patients with high-risk pathology at prostatectomy. Study data were collected and managed using the REDCap electronic data capture platform hosted at Vanderbilt University.18 Our study cohort comprised 1,033 patients that underwent either radical retropubic prostatectomy (RRP) or robotic assisted laparoscopic prostatectomy (RALP) between January 2000 and June 2009 with at least 6 months of follow-up, who on pathologic analysis, were found to have either pathologic T3N0 disease (with or without PSM) or T2N0 disease with PSM. Patients with persistent PSA elevation, defined as PSA ≥0.1 ng/mL on first surveillance PSA ≥ 42 days from surgery or PSA ≥ 0.1 ng/mL on first surveillance PSA < 42 days from surgery if second surveillance PSA was still elevated between 42 to 90 days from surgery, were excluded (N = 134). Patients who underwent adjuvant radiotherapy +/- androgen deprivation therapy were also excluded (N = 25) to limit the likelihood of confounding by indication. After excluding the aforementioned patients, the study comprised 893 patients with either T3N0 disease or T2N0 disease with PSM.
Prior to surgery, all patients underwent a digital rectal examination, PSA testing, and TRUS-guided biopsy with subsequent review of relevant biopsy material by Vanderbilt University Medical Center (VUMC) pathologists. Patients underwent either radical retropubic prostatectomy (RRP) or robotic-assisted laparoscopic prostatectomy (RALP) at our institution. Specimens were submitted in their entirety. External aspects (surgical margins) were inked. Subsequently, the prostate was fixed in neutral buffered formalin, and the apical and bladder neck margins were removed and radially sectioned. The remainder of the prostate was serially sectioned from apex to base, and representative sections (with any suspicious tumor nodule in its entirety) were submitted in conventional tissue blocks to include at least one entire full thickness section from apex, mid and base, seminal vesicle at interface with posterior prostatic tissue, and vas deferens. Pelvic lymph node dissections were examined grossly and all lymphoid tissue was submitted for histological examination. 5-micron hematoxylin-eosin—stained slides were obtained for histologic review. A positive surgical margin was defined as the presence of tumor at the inked margin. For the purpose of this study, margin status was considered as a categorical variable and no consideration was given to the margin line length, focality, or location of the positive margin.
Patients received follow-up care at the discretion of the treating urologist. Most commonly, PSA was collected every 6 months for the first two years, then annually thereafter. The date of last follow-up was defined as the date of the last available PSA value. Biochemical recurrence was defined as PSA >0.2 ng/mL confirmed with a second measurement, or if a patient received secondary treatment (radiation, hormone therapy or chemotherapy) in the setting of a rising PSA. Clinical recurrence was defined as recurrent local disease palpable on exam, or metastatic disease to bone, lymph nodes or other radiographically-detected sites of disease. Those not experiencing recurrence, including those who died of other causes, were censored at the date of the last available PSA. One patient, reported as dead of prostate cancer with no documentation to support evidence or time of recurrence prior to death, was considered a treatment failure at the date of death.19
Statistical Analysis
Baseline clinical and demographic parameters were reported using relevant descriptive statistics. Estimates of the probability of freedom from recurrence were calculated by the Kaplan-Meier method. Candidate predictor variables were fit into a multivariable Cox proportional hazards regression model predicting likelihood of recurrence, which included age, preoperative PSA, pathologic Gleason score, and the presence of EPE, SVI or PSM. Age was included as a continuous variable. Preoperative PSA had a skewed distribution and a suspected nonlinear effect, so it was modeled in a log-transformed fashion.20 Gleason sum was collapsed into four categories ≤6, 7(3+4), 7(4+3) and 8-10. EPE, SVI and PSM were all considered as dichotomous variables. The relationships between candidate covariates were assessed for data reduction to prevent overfitting.20 All statistical analyses were done with R (Version 2.15.1, R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org), with additional functions (called “rms” and “Hmisc”) added. A P value of < 0.05 was considered statistically and all statistical tests were two-sided.
Results
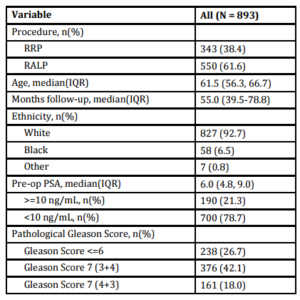
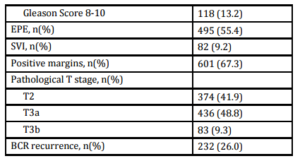
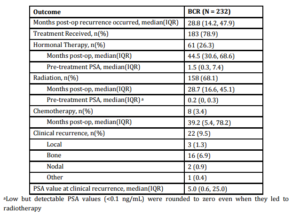
A total of 893 patients met our inclusion criteria, and their demographic, clinical and pathologic characteristics are summarized in Table 1. Median patient age was 61.5 (IQR 56.3-66.7), median pre-operative PSA was 6.0 ng/mL (IQR 4.8-9.0) and median followup was 55.0 months (IQR 39.5-78.8). There were 374 (41.9%) patients with pathologic pT2 disease with PSM, 292 (32.7%) with pT3 disease with negative margins, and 227 (25.4%) with pT3 disease with PSM. In total, 232 (26.0%) patients experienced BCR a median 28.8 months (IQR 14.2-47.9) after prostatectomy. Of those, 183 (78.9%) received treatment during the study period; 158 (68.1%) underwent radiotherapy, 61 (26.3%) received hormone therapy and 8 (3.4%) received chemotherapy. Of those sustaining BCR, 22 (9.5%) experienced clinical recurrence, with the vast majority manifesting bony disease (Table 2).
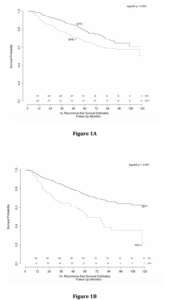
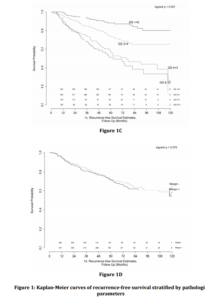
Univariate analysis revealed significant associations between pre-operative PSA, Gleason Score, and the presence of EPE and SVI with time to BCR (Table 3). Multivariable analysis revealed similar relationships, with pre-operative PSA (HR 1.15, 95% CI 1.01-1.31), Gleason 7 (3+4) (HR 2.11, 95% CI 1.36-3.27), Gleason 7 (4+3) (HR 3.81, 95% CI 2.37-6.10), Gleason 8-10 (HR 4.34, 95% CI 2.65-7.11), EPE (HR 1.36, 95% CI 1.01-1.85) and SVI (HR 1.62, 95% CI 1.13-2.33) remaining independently associated with the risk of BCR. Interestingly, surgical margin status failed to achieve statistical significance in either univariate or multivariate analysis. Kaplan-Meier survival analyses revealed less favorable BCR-free survival in patients with EPE (Figure 1a), SVI (Figure 1b) and Gleason 7-10 tumors (Figure 1c) when compared to Gleason 5-6 tumors. There was no difference in survival curves between patients with and without PSM (log-rank = 0.575, Figure 1d).
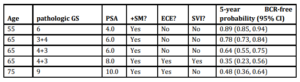
We applied our model to predict the likelihood of recurrence after treatment for different sub-groups of patients. For example, whereas a 65 year old man with a pre-operative PSA of 4 ng/mL, GS 6, no SVI, no ECE, and a PSM has a 5-year BCR-free survival estimate of 90.0%, a 55 year old man with a pre-operative PSA of 10 ng/mL, GS 8, SVI, ECE and a PSM has a 25.0% 5-year BCR-free survival estimate. We have presented a number of clinical scenarios with the point estimates for 5-year BCR-free survival in Table 4.
Discussion
We investigated predictors of recurrence in a large, single-institution, contemporary cohort of 893 patients with high-risk pathologic features after radical prostatectomy who would be considered for early adjuvant radiation therapy. The cohort was comprised of 42% pT2N0/PSM and 58% pT3N0 patients, of which 26% experienced BCR. We were able to identify common clinicopathologic predictors of BCR including PSA, Gleason Score, and the presence of ECE and SVI. It is our hope that, after further validation, the implementation of this risk-stratification tool will optimize the use of early radiation therapy in patients with high-risk features after radical prostatectomy.
Several studies of similar risk groups have reported BCR rates of 30-49%.21-22 Variation in the reported rates of BCR likely reflect differences in the distribution of prostate cancer risk, differences in the definition of BCR and differences in the length of follow-up. Defining what precisely constitutes “high-risk” pathology has remained a variable in the urologic literature and has resulted in considerable challenges in data interpretation and risk-stratification.23 We simultaneously examined the prognostic contribution of multiple clinicopathologic parameters to long-term BCR and clinical outcomes. Multivariable analysis showed GS to be most predictive of BCR, followed by SVI then EPE.
Many investigators have studied predictors of BCR, although most of these studies included all pathologic stages,6,11,23 included only PSM patients,22 or stratified only by clinical parameters.21,24 Alkhateeb et al reported on a series of 1, 268 patients from the University of Toronto to describe the prognostic importance of PSMs.13 Their cohort was comprised of predominantly (67.3%) pT2 patients with a median follow-up of 79 months. The investigators found Gleason Score >8 to be highly predictive of BCR (HR 10.73, 95% CI 4.08-28.21). While supporting the overall trends reported in the current study, Alkhateeb’s study included a high proportion of patients with organ-confined, margin-negative disease, patients that we would not consider for early adjuvant radiation therapy. We sought to include only patients that one might consider for early postoperative RT. By excluding patients with organ-confined margin-negative disease, we built a cohort to reflect the patient population in whom we feel that this model is most applicable.
The importance of PSMs is routinely debated, and our study found that, within this high-risk cohort, the presence of PSMs did not add predictive discrimination. When analyzed among all risk groups post-prostatectomy, PSMs have consistently been identified as risk factors for BCR.4,6,11-13,22-23 Concoran et al found the predictive effect of PSM on BCR was only significant in intermediate risk patients.25 Furthermore, while PSMs are likely to carry discriminative power in a diverse cohort, our inclusion of only those with high-risk pathology (T2R1 or T3RX) likely attenuates the predictive power of margin status. Subgroup analyses from two prospective studies identified PSM as predictors of benefit from aRT.15-16 These results suggest that while PSMs do not predict BCR in this high-risk group, they do portend a favorable response to radiotherapy in those who suffer BCR, presumably because this subgroup is more likely to exhibit local, as opposed to distant, recurrence.
Three randomized controlled trials have investigated the role of aRT in high-risk patients. Each of these studies (SWOG 8794, EORTC 22911 and ARO 96-02) demonstrated improvements in rates of BCR-free survival in patients with variably defined adverse pathologic features.14-16 Of the 3 studies, only SWOG 8794 revealed improvements in both metastases-free and overall survival secondary to adjuvant radiotherapy.14 Despite these benefits, patients in the SWOG cohort who received aRT suffered worse bowel and urinary symptoms than those who received surgery alone.26 Interestingly, aRT did not affect rates of erectile dysfunction or other general health-related quality of life measures. One interpretation of these publications is that all patients with pT3 disease or pT2 with PSM should undergo aRT. However, there may be sub-groups at sufficiently low risk for recurrence in whom initial observation would be a reasonable alternative. Unfortunately, however, the RCTs did not stratify the results by
pathologic GS, or include a sufficient number of pT2 PSM patients to inform the judicious use of aRT in this heterogenous population. By studying a larger population with a wide range of pathologic findings, albeit in a retrospective observational design, we were able to identify risk factors for recurrence, in an effort to develop a more effective risk-stratification scheme that could optimize benefit and minimize harms associated with early radiation therapy.
The clinical implications of this risk stratification strategy are further supported by data comparing aRT to observation followed by early salvage radiotherapy (eSRT).27 Briganti et al conducted a retrospective propensity score-matched cohort study comparing 390 cases of aRT to 500 cases of eSRT, defined as salvage radiotherapy administered at <0.5 ng/mL. The study identified no difference in 5-year BCR-free survival rates, although their population included only pT3N0 patients. Similar to the current study, multivariable analysis revealed pre-operative PSA, pathologic GS and pathologic T-stage to be significant predictors of BCR, while PSM failed to reach significance. While these results, among others, suggest equivalence between adjuvant and early salvage radiation therapy, there remain few high-quality prospective data that specifically address the survival implications of salvage versus adjuvant approach. However, there is currently a randomized-controlled trial underway in the UK to determine the optimal timing of post-operative radiotherapy.28
There are limitations and strengths of the current study that must be considered when interpreting these data. The study cohort includes patients treated by multiple surgeons with non-standardized follow-up protocols and may introduce some variation into our results. Our definition of BCR as >0.2 ng/mL, while consistent with the aforementioned prospective studies, could bias results when compared to groups that set a cut-off point at 0.4 ng/mL.6,11,13 We excluded cases that received aRT, thus some patients at high risk for recurrence were excluded from our analysis. While their outcomes are not represented, they constituted only a small proportion of the population of interest (N = 25). As our cohort spans a ten-year period and multiple pathologists, there is inherent variability in pathologic reporting. Additionally, our data do not include more granular data surrounding positive margins such as line length, number, and Gleason Score at the margin. Finally, while our median follow-up of 55.0 months is comparable to other studies, this analysis would not capture the roughly 20% of pT3 patients that recur after 5-years.14 Nonetheless, our study had a sufficient number of patients with an adequately wide range of recurrence risk, and over 5 years median follow up to allow for the identification of risk factors for recurrence and development of a model to predict recurrence in different sub-groups of patients.
Conclusion
Patients with adverse pathologic features represent a heterogenous group with variable natural histories. Certainly, the definition of “high risk” varies for each patient, and further delineating an individual’s risk profile will facilitate shared decision-making. This study highlights that heterogeneity and our findings suggest that incorporating a personalized risk stratification scheme may assist patients and physicians with decision making to optimize the benefits and minimize the harms of radiotherapy following surgery for prostate cancer.
Figures and Tables
Table 1: Cohort Characteristics
Table 2: Outcomes of patients with biochemical recurrence
Table 3: Univariate analysis and multivariable cox proportional hazards model of predictors of BCR
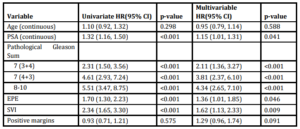
Table 4: Clinical scenarios predicting 5-year BCR free survival using the multivariable model
Figure 1: Kaplan-Meier curves of recurrence-free survival stratified by pathologic parameters
Acknowledgment
The REDCap database is supported by Grant UL1 TR000445 from NCATS/NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. MJR was supported by the Veterans Affairs National Quality Scholars Program with use of facilities at Veterans Health Administration Tennessee Valley Healthcare System and by a grant from the Urology Care Foundation Research Scholars Program and the AUA Southeastern Section Research Scholar Fund.
References
1.Siegel R, Naishadham D and Jemal, A. (2013) “Cancer statistics,” CA Cancer J Clin, 63 (1) 11-30.
Publisher – Google Scholar
2.Cooperberg MR, Broering JM and Carroll PR. (2010) “Time trends and local variation in primary treatment of localized prostate cancer,” J Clin Oncol, 28 (7) 1117-1123.
Publisher – Google Scholar
3.Roehl KA, Han M, Ramos CG, et al. (2004) “Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results,” J Urol, 172 (3) 910-914.
Publisher – Google Scholar
4.Han M, Partin AW, Zahurak M, et al. (2003) “Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer,” J Urol, 169 (2) 517-523.
Publisher
5.Pinto F, Prayer-Galetti T, Gardiman M, et al. (2006) “Clinical and pathological characteristics of patients presenting with biochemical progression after radical retropubic prostatectomy for pathologically organ-confined prostate cancer,” Urol Int, 76 (3) 202-208.
Publisher
6.Swindle P, Eastham JA, Ohori M, et al. (2008) “Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens,” J Urol, 179 (5) 847-851.
Publisher
7.Swanson GP, Riggs M, Hermans M. (2007) “Pathologic findings at radical prostatectomy: risk factors for failure and death,” Urol Oncol, 25 (2) 110-114.
Publisher
8.Blute ML, Bergstralh EJ, Iocca A, et al. (2001) “Use of Gleason score, prostate specific antigen, seminal vesicle and margin status to predict biochemical failure after radical prostatectomy,” J Urol, 165 (1) 119-125.
Publisher
9.Barocas DA, Salem S, Kordan Y, et al. (2010) “Robotic assisted laparoscopic prostatectomy versus radical retropubic prostatectomy for clinically localized prostate cancer: comparison of short-term biochemical recurrence-free survival,” J Urol 183 (3) 990-996.
Publisher
10.Eastham JA, Kattan MW, Riedel E, et al. (2003) “Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens,” J Urol 170 (6) 2292-2295.
Publisher
11.Karakiewicz PI, Eastham JA, Graefen M, et al. (2005) “Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients,” Urology 66 (6) 1245-1250.
Publisher
12.Wright JL, Dalkin BL, True LD, et al. (2010) “Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality,” J Urol 183 (6) 2213-2218.
Publisher
13.Alkhateeb S, Alibhai S, Fleshner N, et al. (2010) “Impact of positive surgical margins after radical prostatectomy differs by disease risk group,” J Urol 183 (1) 145-150.
Publisher
14.Thompson IM, Tangen CM, Paradelo J, et al. (2009) “Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial,” J Urol 181 (3) 956-962.
Publisher
15.Wiegel T, Bottke D, Steiner U, et al. (2009) “Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95,” J Clin Oncol 27 (18) 2924-2930.
Publisher
16.Bolla M, Van Poppel H, Tombal B, et al. (2012) “Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911),” Lancet 380 (9858) 2018-2027.
Publisher
17.Grossfeld, GD, Tigrani VS, Nudell D, et al. (2000) “Management of a positive surgical margin after radical prostatectomy: decision analysis,” J Urol 164 (1) 93-99.
Publisher
18.Harris PA, Taylor R, Thielke R, et al. (2009) “Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support,” J Biomed Inform 42 (2) 377-381.
Publisher
19.Stephenson AJ, Scardino PT, Eastham JA, et al. (2005) “Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy,” J Clin Oncol 23 (28) 7005-7012.
Publisher
20.Harrell FE Jr. (2001) Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer-Verlag, New York, NY, USA.
Publisher
21.Grossfeld GD, Latini DM, Lubeck DP, et al. (2003) “Predicting recurrence after radical prostatectomy for patients with high risk prostate cancer,” J Urol 169 (1) 157-163.
Publisher
22.Resnick MJ, Canter DJ, Guzzo TJ, et al. (2010) “Defining pathological variables to predict biochemical failure in patients with positive surgical margins at radical prostatectomy: implications for adjuvant radiotherapy,” BJU Int 105 (10) 1377-1380.
Publisher – Google Scholar
23.Walz J, Joniau S, Chun FK, et al. (2011) “Pathological results and rates of treatment failure in high-risk prostate cancer patients after radical prostatectomy,” BJU Int 107 (5) 765-770.
Publisher – Google Scholar
24.Loeb S, Schaeffer EM, Trock BJ, et al. (2010) “What are the outcomes of radical prostatectomy for high-risk prostate cancer?” Urology 76 (3) 710-714.
Publisher
25.Corcoran NM, Hovens CM, Metcalfe C, et al. (2012) “Positive surgical margins are a risk factor for significant biochemical recurrence only in intermediate-risk disease,” BJU Int 110 (6) 821-827.
Publisher – Google Scholar
26.Moinpour CM, Hayden KA, Unger JM, et al. (2008) “Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy,” J Clin Oncol 26 (1) 112-120.
Publisher
27.Briganti A, Wiegel T, Joniau S, et al. (2012) “Early Salvage Radiation Therapy Does Not Compromise Cancer Control in Patients with pT3N0 Prostate Cancer After Radical Prostatectomy: Results of a Match-controlled Multi-institutional Analysis,” Eur Urol 62 (3) 472-487.
Publisher
28.Parker C, Clarke N, Logue J, et al. (2007) “RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery),” Clin Oncol (R Coll Radiol) 19 (3) 167-171.
Publisher