Introduction
Diabetes is a chronic illness, which not only has a range of daily treatment demands, but also carries the risk of major health complications in later life (American Diabetes Association, 2012). Families where a young person has type 1 diabetes are presented with major challenges on several fronts. Effective control of diet, and management of regular medication are constantly needed, with the consequences if these are not in balance being an unrelenting worry. The pressure of these issues is increased by the recognition that the maintaining of optimal glycaemic control is crucial in order to prevent or delay the potentially serious health complications associated with the condition (Healthcare Quality Improvement Partnership, 2011).
Given the constant strain, these issues must prompt, it would not be surprising to find parents develop symptoms of stress and even frank mental illness, such as depression. The research has shown that some parents experience a decrease in the level of enjoyment they gain from being a parent because of the worry (Northam, Todd and Cameron, 2006), and this is especially so if there is a conflict about the management of the diabetes with the young person (Williams, Laffel and Hood, 2009), and worry that the young person may have a hypoglycaemic episode (Streisand, Swift, Wickmark, et al.,2005).
Although the research does not indicate that having a child with diabetes can directly prompt mental health illnesses such as depression (Quittner, Espelage, Opipari, et al.1998; Silver, Westbrook, and Stein, 1998), it does tend to place them at an increased risk (Cohen, 1999; Jaser, Whittemore, Ambrosino, et al., 2008). This can be mitigated to some degree by a well organised and effective management regime which produces better glycaemic control, improves adherence to treatment, and thus, results in less family conflict (Anderson, 2004). As the focus of this parental concern and structured management regime, the young person is likely to be receiving a pattern of parenting somewhat different from their peers. This, together with the young person’s own concerns about their well-being and developmental challenges, can make the maintenance of optimal control difficult (Ingerski, Anderson, Dolan, et al., 2010). There is some conflicting evidence about the prevalence of frank emotional and behavioural disorders in young people with type 1 diabetes (Bryden, Peveler, Stein,et al., 2001: Northam,
Matthews, Anderson, et al., 2005), with the prevalence of depression; for instance, being reported as far more common than in the general population of adolescents by some (Gray, Whittemore and Tamborlane, 2002), but not others (Lawrence, Standiford, Loots, et al., 2006).
Studies in this area have found that female adolescents with diabetes are at an increased risk of developing an eating disorder (Jones, Lawson, Daneman, et al., 2000), and boys with diabetes are more likely to show disruptive behaviour disorders (Goldston, Kelley, Reboussin, et al., 1997). Overall, it would appear that having type 1 diabetes does confer a somewhat increased risk of developing a mental health problem [20], but what is not in doubt, is the impact of having mental health problems can have upon the glycaemic control [Rewers et al., 2002; Naar-King, et al., 2004; Northam et al., 2005; Cohen, Lumley, Hassan, Loar, Anderson, et al., 2006; Skocić, Rudan, Brajković, et al., 2009). Glycaemic control is judged by measuring the amount of glucose adhering to red blood cells (HbA1c), with a HbA1c level of less than 7.5% (58mmol/mol) being recommended as giving good glycaemic control (National Institute of Clinical Excellence, 2005), butthe majority of adolescents with type 1 diabetes do not achieve these optimal levels (Ingerski et al., 2010).
However, studies on the adverse interaction between mental health difficulties and glycaemic control have tended to focus upon frank mental health problems (Blanz, Rensch-Riemann, Fritz-Sigmund, et al., 1993; Kovacs, Goldston, Obrosky, et al., 1997), and relatively severe disruptions to control, such as recurrent diabetic ketoacidosis (Liss, Waller, Kennard, et al., 1997). Although such issues are well- represented in the literature, there is little known about the impact of sub-optimal diabetic control when major disruption is not evident. To explore this, a sample of young people with type 1 diabetes where professional concern was not high was recruited, and issues of family functioning and mental health explored.
Methods
The study sample was made up of young people between the age of 9 to 16 years who had a diagnosis of type 1 diabetes mellitus, and were currently attending a specialist paediatric diabetes clinic in the North East of England, (five clinics in total participated). All clinic attenders within the age range were considered for inclusion. The aim of the study was to explore the issues in routine clinic attenders who were not presenting any concerns, and to this end, families were approached where there were no major concerns about the day-to-day management of the diabetes, no issues around poor behaviour, and the index child had no significant co-morbid medical condition. Families where a member had a serious physical illness, severe psychopathology (e.g. psychosis), or significant learning disability were also not considered for inclusion. Young people who had an average HbA1C over the last year of <7.5 % (9% (> 75mmol/mol) were recruited to the suboptimal group.
Having obtained ethical approval from academic and health bodies, written consent was sought from all participating parents and their children, and data were gathered by the diabetic nursing team about the family demographics, and the child’s diabetic history and care. In addition, the mothers of the young people were asked to complete the General Health Questionnaire (GHQ) which is a widely used measure aimed at detecting short-term psychiatric disturbance, and is the ‘scaled’ version of the original GHQ. The measure is designed for use with individuals over 11 years of age. The scale is divided into four sub-scales: Somatic symptoms, Anxiety/Insomnia, Social dysfunction, and severe depression. Each sub-scale contains 7 items. Each participating parent/carer was asked to complete the GHQ-28 based on their general health over the past few weeks. In terms of validity, sensitivity values ranging from 44% to 100% have been identified, and specificity values ranging from 74% to 93% (Goldberg et al., 1997). The Cronbach’s α for internal consistency ranges from 0.77 to 0.93 (Failde, Ramos and Fernandez-Palacin, 2000), with inter- and intra-rater reliability being excellent (0.9 — 0.95) (Failde et al., 2000), and test-retest reliability ranging from 0.78 to 0.90 (Robinson and Price, 1982). For identifying caseness, the total score of the sub-scales is used.
The mothers were also asked to fill in the Swanson, Nolan and Pelham Questionnaire (SNAP IV) which consists of 26 items, and is made up of two sub-sets of symptoms from the DSM-IV criteria for Attention Deficit Hyperactivity Disorder (ADHD): inattention (items 1 to 0) and hyperactivity/impulsivity (items 10 to 18). It also has 8 questions relating to oppositional symptoms. The items are rated on a 4-point scale from (0) not at all to (3) very much, with the two ADHD subscores being added together to give a total ADHD score. The scale has been used in many treatment studies (e.g.MTA Cooperative Group, 1999; Swanson, Gupta, Lam, et al., 2003; Correia Filho, Bodanese, Silva, et al., 2006), and in a recent re-analysis [38], the coefficient alpha for overall parent ratings was .94, with the inattentive and hyperactive/impulsive subdomains being .90 and .79 respectively.
The Ontario Child Health Scale (OCHS) is a standardised instrument composed of 112 items, designed to measure the emotional and behavioural functioning of youngsters aged 6 to 16 years. Each participating mother was asked to complete the OCHS in relation to their child. The OCHS is a standardised instrument composed of 112 items, designed to measure the emotional and behavioural functioning of youngsters aged 6 to 16 years. Original validation confirmed the accuracy of the tool in mapping to DSM III-R diagnostic categories (Conduct Disorder, Oppositional Disorder, Attention-Deficit Hyperactivity Disorder, Overanxious Disorder, and Depression) (Boyle et al., 1993). Test – retest reliability on the subscales ranges from 0.65 to 0.84, and all internal consistency and reliability estimates for the scale exceed 0.7, except for the Conduct Disorder scale for parents ratings of 6 to 11 year olds (α = 0.68) (Boyle et al., 1993). The sub-scores for Conduct Disorder, Oppositional Disorder, Over Anxious and Depression were used in this study. The measure has been validated for a North East of England population (Place, Martin, Hildreth, et al., 1999), and accuracy in mapping to DSM IV diagnostic categories, as well the convergent and discriminant validity of the measure, having been further confirmed (Bussing, Fernandez, Harwood, et al., 2008).
The Family Adaptation and Cohesion Evaluation Scales (FACES IV) is a self-report profile scoring system, allowing the scale scores to be interpreted as separate assessments of family functioning. Percentile scores for the six scales can be obtained in order to determine which cluster the family profile most closely approximates (Balanced, Rigidly Cohesive, Midrange, Flexibly Unbalanced, Chaotically Unbalanced and Unbalanced). The scoring system also allows compilation and comparison of scores for a given family system. A ratio score is obtainable for the scales of cohesion (cohesion ratio) and flexibility (flexibility ratio), as well as for the two scales combined (Total Circumplex Ratio). The higher the ratio score above 1, the more balanced the family system; and the lower the ratio score below 1, the more unbalanced the system. The ratio score enables a families relative strength, and problem areas to be summarised into a single score. Percentile scores can also be obtained for the Family Communication and Family Satisfaction scales, as well as a total percentile score for these two scales, with higher scores indicating more positive feelings about family communication and satisfaction. Reliability of the FACES IV scales has been found to be acceptable for research and clinical purposes, with Cronbach’s α values for the scales and subscales being; Cohesion, = 0.89, Flexibility, = .0.84, Rigid = 0.82, Enmeshed = 0.77, Disengaged = 0.87, and chaotic = 0.86 (Boyle, et al., 1993). It has also been shown to discriminate between healthy and problematic family functioning (Olson and Gorall, 2003).
All statistical analyses were conducted with SPSS version 21.0 (SPSS for Windows Inc., Chicago, IL, USA). For significance tests, alpha was set at 0.05, and a Bonferroni Correction was applied to calculations using multiple scale correlation to correct for Type I error inflation.
66 families agreed to take part in the study; two did not fully meet the criteria for inclusion because of wider health concerns. The final study comprised of 37 boys with a mean age 12.9 years (range 10 — 16.5 years), and 27 girls with a mean age 13.3 years (range 9.8 — 16.6 years). The mean duration of type 1 diabetes for the sample was 5.5 years.
Results
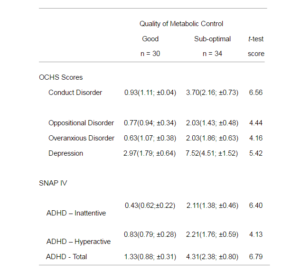
Of the 37 boys, 19 had sub-optimal metabolic control, and of the 27 girls, 15 did not have good control. The mean ages and the mean duration of diabetes were not statistically significant between the groups. Considering the whole sample, the analysis of the OCHS and SNAP-IV scales (Table 1) showed that young people with sub-optimal control had a significantly higher level of symptoms on all scales compared to those with good control. Using the published cut-off scores for caseness (Goldberg et al., 1997; Steele, Weiss, Swanson, et al., 2006), no subjects had a score on the SNAP-IV that approached clinical levels, but one girl was above the cut-offs for depression and conduct disorder, with a second girl over the cut-off for being overanxious. With regard to the boys, three reached the cut-off score for depression, all of whom were in the sub-optimal glycaemic control group.
Table 1: Comparison of the Means (SD; Confidence Interval) Scores on the OCHS, and SNAP IV in the Good and Sub-optimal Control Groups, and Results of t-test Analysis (with df = 62, all Significant to p< 0.0001
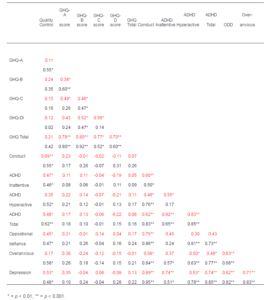
Constructing a correlation matrix to calculate the Pearson Product-Moment Correlation Coefficients from the results (Table 2) confirmed clear statistically significant associations between the sub-optimal glycaemic control and the increased emotional and behavioural symptoms for both boys and girls, except for being overanxious, which was not found to have a significant association for girls.
Table 2 — Correlation Matrix (Pearson Product-Moment Correlation Coefficient) of Young People’s Mental Health Results and Their Mother’s GHQ-28 Scores (n= 51). (Boys Results (n=37) Shown in , and Girls (n=27) Shown in )
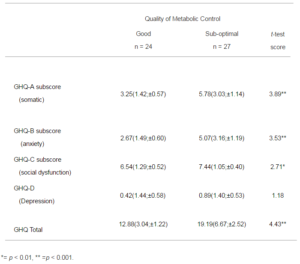
13 of the mothers failed to complete the GHQ-28 questionnaire because the fathers managed clinic attendance. There was no statistically significant difference in the quality of diabetic control, when these families were compared to those where the mothers did complete the GHQ (m = 8.77, sd = 1.56, and m = 8.67, sd = 1.59). The results from this scale (Table 3) show significantly increased levels of symptoms in the mothers with young people who are showing sub-optimal control except for the depression subscale. The most significant difficulties are evident in somatic symptoms and anxiety, but this is only in the mothers of boys. A score of 24 or above indicates caseness in this scale (Liss et al., 1998), and 6 of the mothers are scored in this range.
Table 3: Comparison of the Mothers GHQ-28 Means (SD; Confidence Interval) in Good and Sub-optimal Control Groups, and Results of t-test Analysis (with df = 49)
The analysis of the association between the subscales of the GHQ-28 and gender (Table 2) show that for mothers of boys the somatic symptoms subscale has a strong association with anxiety, but not with social dysfunction or depression, while anxiety symptoms do. For the mothers of girls the association between somatic symptoms and anxiety is less evident, while the other subscales show significant correlation between them. There is no statistical correlation between the GHQ results and the young people’s emotional and behavioural symptoms.
The assessment of the family functioning through the FACES IV questionnaire permitted associations between this and the young people’s mental health symptoms, and the mother’s GHQ-28 scores to be examined (Table 4). The maternal perceptions of the family functioning revealed no associations with girls symptoms, but for boys oppositional defiance and conduct problems tended to be associated with poorer family communication, and showing some ADHD symptoms with the degree of satisfaction with family life, the statistical significance of these results being lost when the Bonferroni correction was applied.
Table 4 — Correlation Matrix (Pearson Product-Moment Correlation Coefficient) of Young People’s Mental Health Results and Their Mother’s GHQ-28 Scores Compared to Their Responses on the FACES IV Questionnaire. (Boys Results (n=29) Shown in  , and Girls (n=22) Shown in
, and Girls (n=22) Shown in  )
)
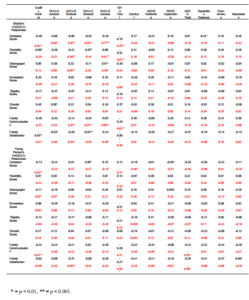
The young people’s responses on the FACES IV (Table 4) showed some gender differences. The girls reported issues with family cohesion, communication, and satisfaction with family life if they were showing appreciable externalising behaviour, but no issues if their symptoms were emotional in nature. The boys with oppositional defiance and ADHD symptoms also tended to report a poorer sense of family cohesion, but it was emotional symptoms, not externalising ones, that were associated with the sense of satisfaction with family life.
The maternal GHQ-28 scores showed marked association with their sense of family cohesion and flexibility, the quality of family communication, and to a lesser degree feeling disengaged from the family, but only in the mothers of girls. For the mothers of boys, it was the association with their sense of satisfaction with family life that was the most striking.
Discussion
Adolescence is a difficult phase of development for any family to traverse, and if the young person has an enduring and potentially life-threatening illness this compounds the difficulty. It is well-recognised that adolescence brings an increase in symptoms of emotional upset and externalising behaviours (Fatori, Bordin, Curto, et al., 2013), as well as increases in concerning behaviours such as deliberate self-harm (Wilkinson, 2013). In considering adolescents with type 1 diabetes, the research evidence suggests that emotional difficulties are associated with an increased risk of poor glycaemic control (Rewers, et al., 2002; Cohen, et al., 2004; Northam, et al., 2005; Bernstein, Stockwell, Gallagher, et al., 2013), and this increases the risk of health complications emerging (Diabetes Control and Complications Trial Research Group, 1993). While, the impact on general health of sub-optimal glycaemic control (such as deteriorating eyesight and peripheral vascular difficulties that can necessitate limb amputation) are clearly of major importance, there is growing evidence that more subtle effects upon brain functioning are also occurring (Nylander, Toivonen, Nasic, et al., 2013). The brain requires significant levels of glucose to function, and since it can neither synthesize nor store glucose; it is an organ that is very dependent upon glucose regulation being effectively managed (Boyle, Nagy, O’Connor, et al., 1994). Fluctuating glucose levels can disrupt various brain processes, giving rise to structural alterations and changes in brain function (Gispen and Biessels, 2000; Perantie, Koller, Weaver, et al., 2011; Antenor-Dorsey, Meyer, Rutlin, et al., 2013).
The impact of such effects can be seen in several brain structures, with one of the most significant being hippocampus. This has an important role in integrating learning and memory, with frequent hypo-glycaemic episodes predicting greater memory problems than found in controls (Hershey, Perantie, Warren, et al., 2005). However, these deficits are often modest, not placing the subjects in the range that prompts major clinical concern (Brands, Kessels, Hoogma, et al., 2006), but rather increasing the general level of difficulties. In addition, the hippocampus has a role in regulating emotional control (Fanselow and Dong, 2010), and the management of anxiety (Barkus, McHugh, Sprengel, et al., 2010). The hippocampus has been found to be extremely sensitive to changes in glucose levels, with fluctuations being associated with decreased hippocampal neurogenesis (Gispen and Biessels, 2000). It has been postulated that this is a possible mechanism to explain how emotional difficulties that are associated with sub-optimal glycaemic control, such as depression, arise (Lyoo, Yoon, Jacobson, et al., 2012). The findings in this study would be consistent with such an assertion. The sample was chosen because they were not showing symptoms which were prompting major clinical concern, but nevertheless if a young person had sub-optimal glycaemic control, they were more likely to show more emotional symptoms and externalising behaviours than their peers.
Work in this field has repeatedly shown that sub-optimal glycaemic control is associated with depressive symptoms (Monaghan, Singh, Streisand, et al., 2010; Cameron and Northam, 2012), with a potential mechanism being the disturbance of hippocampal functioning (Boyle et al., 1994) and/or cortical architecture (Lyoo, Yoon, Jacobson, et al.,2012). In the current study, there was a clear increase of depressive symptoms among young people, and while this sample cannot be considered representative of all adolescents with type 1 diabetes, it is interesting that more boys were found to be struggling with significant symptoms of depression than girls, a reversal of the prevalence in the general population (Essau, Lewinsohn, Seeley, et al., 2010). These young people were not previously thought to be depressed, and this finding emphasizes the importance of routine screening for mental health, and family difficulties to ensure that any changes in these young people’s mental health are detected as early as possible.
There was also an increase in anxiety symptoms among the sub-optimal control group, which may not be altogether surprising given the major health issues that are associated with type 1 diabetes. Anxiety symptoms may not be evident in clinic unless specifically sought, but such anxiety can be a significant issue, not only as a disorder in its own right, but also because of its impact upon the care and management of the diabetes more generally (Sinnamon, Caltabiano and Baune, 2013).
This study found that, as well as an increase in emotional symptoms, there was also an increased level of externalising behaviour if the glycaemic control was sub-optimal. This has been recognised as a significant influence upon the quality of glycaemic control, with externalising symptoms tending to exacerbate parent—adolescent conflict and hence, reducing cooperation over treatment (Luyckx, Seiffge-Krenke, Missotten, et al., 2013). The results presented here support such a view, with the more externalising behaviour symptoms being associated with the adolescents viewing family communication as poorer than their peers.
A particular form of externalising behaviour is ADHD, and symptoms associated with ADHD were more evident in the sub-optimal group, though none reached the level sufficient for diagnosis. There is some suggestion of an association between type I diabetes in children and ADHD symptoms (Chen, Lee, Yeh, et al., 2013), and the changes in the prefrontal cortex associate with sub-optimal glycaemic control (Lyoo et al., 2012) may offer an explanation for this link.
Most parents experience some distress after the child is diagnosed with type 1 diabetes [67-69], which can become a persistent distortion of functioning (Whittemore, Urban, Tamborlane, et al., 2003; Helgeson, Becker, Escobar, et al.,2012). Indeed, maternal depressive symptoms are one of the strongest risk factors for predicting the young person’s own mental health (Kovacs et al., 1997; Jasser et al., 2008). In addition, if the mother is over-anxious this can adversely affect both the glycaemic control and the young person’s general functioning still further (Cameron, Young and Wiebe, 2007). In the current study little correlation between the GHQ results and the young people’s emotional and behavioural symptoms was found, although the mothers of girls did show a modest correlation between the girl being anxious and the mother reporting depressive symptoms. As noted, associations between maternal distress and distress in children have been identified in the literature; in particular in relation to children with diabetes it has been found that the higher the distress of mothers, the higher will be the distress of the children (Kovacs et al., 1997). As well as this predictive element of maternal psychological distress, if the mother has depressive symptoms this predicts an increased risk of perhaps 2.6 fold that the child will develop depressive symptoms later in life (Kovacs et al., 1997). As in the current study, a trend within the research literature is that most parents with a chronically ill child do not show clinical levels of depression. However, the increased level of symptoms over the general population may place these parents at an increased risk (Cohen, 1999).
In this study, the mothers of boys with sub-optimal control of their diabetes showed the higher levels of anxiety, and tended to be less satisfied with family life. The mothers of girls with sub-optimal glycaemic control tended to see the family life as less cohesive and flexible. As found in previous research (Cohen et al., 2004; Missotten, Luyckx and Seiffge-Krenke, 2013), the current study found that a positive family environment (as described by a sense of cohesion amongst its members) is strongly associated with the young person achieving the most favourable health outcomes. Cohesion is well recognised as a significant influence upon diabetic control (Anderson, Miller, Auslander, et al., 1981; Mackey, Hilliard, Berger, et al., 2011), it is also associated with parental warmth and the child’s cooperation with their diabetic management (Anderson, 2004). By contrast, adolescents with suboptimal diabetic control tend to view their families as less cohesive (Zashikhina and Hagglof, 2009). As evidence indicates that a well organised and effective management regime aids glycaemic control, improve adherence to treatment, and prompt less family conflict about the diabetes (Seiffge-Krenke, 1998), it is not surprising that a more chaotic approach to family life impacts unhelpfully on the glycaemic control.
The small sample involved in the study is a limitation, and this also meant that the potential impact of a relatively wide age range within the sample could not be explored. However, the choice of a sampling method which selected subjects where their glycaemic control status was clear was made in an effort to give more robust results from the study. While this allowed associations to be explored, it prevented any inference of frequency in wider populations to be made. In addition, the cross-sectional design does not permit inference as to causation, and the relatively close geographical area from which the sample was drawn means any implications for other populations should be made with caution.
Conclusion
There is a growing evidence base about the adverse impact sub-optimal glycaemic control has upon brain structure and function. The results from this study show that even when there are no clear mental health difficulties, sub-optimal glycaemic control is associated with an increase in emotional and behavioural symptoms, which potentially reflects these brain changes. This adds weight to the importance of mental health screening in routine review clinics, and emphasises the need for clinic staff to recognise that sub-optimal glycaemic control is likely to be associated with symptoms of distress, and disruption which may not be plainly evident. Given that any changes in the adolescent period are likely to be life-long, it is crucial that any difficulties are addressed as early as possible to optimise current management, and ensure the future well-being of the young person.
Competing Interests
None known
Acknowledgements
Our grateful thanks to the staff of the paediatric departments of the North East; Diabetes Forum for their untiring cooperation and assistance, and to Ms Lucy Bulmer for her assistance in the preparation of the tables.
References
1.American Diabetes Association (2012). “Diagnosis and Classification of Diabetes Mellitus,” Diabetes Care, 35, S64 – S71.
Publisher – Google Scholar
2.Anderson, B. J. (2004). “Family Conflict and Diabetes Management in Youth: Clinical Lessons From Child Development and Diabetes Research,” Diabetes Spectrum, 17, 22-26.
Publisher – Google Scholar
3.Anderson, B. J., Miller, J. P., Auslander W. F. & Santiago, J. V. (1981).”Family Characteristics of Diabetic Adolescents: Relationship to Metabolic Control,” Diabetes Care, 4, 586-594.
Publisher – Google Scholar
4.Antenor-Dorsey, J. A. V., Meyer, E., Rutlin, J., Perantie, D. C., White, N. H., Arbelaez, A. M., Shimony, J. S. &Hershey, T. (2013). “White Matter Microstructural Integrity in Youth with Type 1 Diabetes,” Diabetes, 62, 581—589.
Publisher – Google Scholar
5.Barkus, C., McHugh, S. B., Sprengel, R., Seeburg, P. H., Nicholas, J., Rawlins, P. & Bannermana, D. M. (2010). “Hippocampal NMDA Receptors And Anxiety: At the Interface between Cognition and Emotion,” European Journal of Pharmacology, 626, 49—56.
Publisher – Google Scholar
6.Benedict, C., Hallschmid, M., Schultes, B., Born, J. & Kern, W. (2007). ”Intranasal Insulin to Improve Memory Function in Humans,” Neuroendocrinology 86, 136- 142.
Publisher – Google Scholar
7.Bernstein, C. M., Stockwell, M. S., Gallagher, M. P., Rosenthal, S. L. & Soren, K. (2013). “Mental Health Issues in Adolescents and Young Adults with Type 1 Diabetes: Prevalence and Impact on Glycemic Control,” Clinical Pediatrics,52, 10-15.
Publisher – Google Scholar
8.Blanz, B. J., Rensch-Riemann, B. S., Fritz-Sigmund, D. I. & Schmidt, M. H. (1993). “IDDM is a Risk Factor for Adolescent Psychiatric Disorders,” Diabetes Care, 16, 1579-1587.
Publisher – Google Scholar
9.Boyle, M. H., Offord, D. R., Racine, Y., Sanford, M., Szatmari, P., Fleming, J. E. & Price-Munn, N. (1993). “Evaluation of the Diagnostic Interview for Children and Adolescents for Use in General Population Samples,” Journal of Abnormal Child Psychology, 21, 663- 681.
Publisher – Google Scholar
10.Boyle, P. J., Nagy, R. J., O’Connor, A. M., Kempers, S. F., Yeo, R. A. & Qualls, C. (1994). “Adaptation in Brain Glucose-Uptake Following Recurrent Hypoglycemia,” Proceedings of the National Academy of Sciences of the United States of America, 91, 9352-9356.
Publisher – Google Scholar
11.Brands, A. M., Kessels, R.P., Hoogma, R. P., Henselmans, J. M., van der Beek Boter, J. W., Kappelle, L. J., de Haan, E. H. & Biessels, G. J. (2006). “Cognitive Performance, Psychological Well-Being, and Brain Magnetic Resonance Imaging in Older Patients with Type 1 Diabetes,” Diabetes, 55, 1800-1806.
Publisher – Google Scholar
12.Bryden, K. S., Peveler, R. C., Stein, A., Neil, A., Mayou, R. A. & Dunger, D. B. (2001). “Clinical and Psychological Course of Diabetes from Adolescence to Young Adulthood: A Longitudinal Cohort Study,” Diabetes Care, 24, 1536—1540.
Publisher – Google Scholar
13.Bussing, R., Fernandez, M., Harwood, M., Hou, W., Garvan, C. W. Eyberg, S. M. & Swanson, J. M. (2008). “Psychometric Properties and Normative Ratings from a School District Sample,” Assessment, 15, 317-328.
Publisher – Google Scholar
14.Cabizuca, M., Margques-Portella, C., Mendlowicz, M.V., Coutinho, E. S. & Figueira, I. (2009). “Posttraumatic Stress Disorder in Parents of Children with Chronic Illnesses: A Meta-Analysis,” Health Psychology, 28, 379—388.
Publisher – Google Scholar
15.Cameron, F. J. & Northam, E. A. (2012). ”Screening for Psychological Disorders in Youth with Type 1 Diabetes: Who, When, What and How?,” Diabetes Management, 2, 513-520.
Publisher – Google Scholar
16.Cameron, L. D., Young, M. J. & Wiebe, D. J. (2007). “Maternal Trait Anxiety and Diabetes Control in Adolescents with Type 1 Diabetes,” Journal of Pediatric Psychology, 32, 733—744.
Publisher – Google Scholar
17.Chen, H. J., Lee, Y. J., Yeh, G. C. & Lin, H. C. (2013).”Association of Attention-Deficit/Hyperactivity Disorder with Diabetes: A Population-Based Study,” Pediatric Research, 73, 492-496.
Publisher – Google Scholar
18.Cohen, M. W. (1999). “Families Coping With Childhood Chronic Illness: A Research Review,” Family Systems & Health, 17, 149-164.
Publisher – Google Scholar
19.Cohen, D. M., Lumley, M. A., Naar-King, S., Partridge, T. & Cakan, N. (2004). “Child Behavior Problems and Family Functioning As Predictors of Adherence and Glycemic Control in Economically Disadvantaged Children with Type 1 Diabetes: A Prospective Study,” Journal of Pediatic Psychology, 29, 171- 184.
Publisher – Google Scholar
20.Correia Filho, A. G., Bodanese, R., Silva, T. L., Alvares, J. P., Aman, M. & Rohde, L. A. (2005). “Comparison of Risperidone and Methylphenidate for Reducing ADHD Symptoms in Children and Adolescents with Moderate Mental Retardation,” Journal of the American Academy of Child & Adolescent Psychiatry, 44, 748-755.
Publisher – Google Scholar
21.Diabetes Control and Complications Trial Research Group, (1993). “The Effect of Intensive Diabetes Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus,”New England Journal of Medicine, 329, 977—986.
Publisher – Google Scholar
22.Essau, C. A., Lewinsohn, P. M., Seeley, J. R. & Sasagawa, S. (2010). ”Gender Differences in the Developmental Course of Depression,” Journal of Affective Disorders, 127, 185-190.
Publisher – Google Scholar
23.Failde, I., Ramos, I. & Fernandez-Palacin, F. (2000). “Comparison between the GHQ-28 and SF-36 (MH 1-5) for the Assessment of the Mental Health in Patients with Ischaemic Heart Disease,” European Journal of Epidemiology, 16, 311-316.
Publisher – Google Scholar
24.Fanselow, M. S. & Dong, H-W. (2010).”Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures?,”Neuron, 65, 7.
Publisher – Google Scholar
25.Fatori, D., Bordin, I. A., Curto, B. M. & de Paula, C. S. (2013). “Influence of Psychosocial Risk Factors on the Trajectory of Mental Health Problems from Childhood to Adolescence: A Longitudinal Study,” BMC Psychiatry, 13, 31.
Publisher – Google Scholar
26.Gispen, W. H. & Biessels, G. J. (2000).”Cognition and Synaptic Plasticity in Diabetes Mellitus,” Trends in Neuroscience, 23, 542-549.
Publisher – Google Scholar
27.Goldberg, D. P., Gater, R., Sartorius, N., Ustun, T. B., Piccinelli, M., Gureje, O. & Rutter, C. (1997). “The Validity of Two Versions of the GHQ in The WHO Study Of Mental Illness In General Health Care,” Psychological Medicine, 27, 191-197.
Publisher – Google Scholar
28.Goldston, D. B., Kelley, A. E., Reboussin, D. M., Daniel, D. B. S. S., Smith, J. A., Schwartz, R. P., Lorentz, W. & Hill, C. (1997). “Suicidal Ideation and Behaviour and Noncompliance with the Medical Regimen among Diabetic Adolescents,”Journal of the American Academy of Child and Adolescent Psychiatry, 36, 1528—1536.
Publisher – Google Scholar
29.Gray, M., Whittemore, R. & Tamborlane, W. (2002). “Depression in Type 1 Diabetes in Children: Natural History and Correlates,” Journal of Psychosomatic Research, 53, 907—911.
Publisher – Google Scholar
30.Hassan, K., Loar, R., Anderson, B. J. & Heptulla, R. A. (2006). “The Role of Socioeconomic Status, Depression, Quality of Life, and Glycemic Control in Type 1 Diabetes Mellitus,” Journal of Pediatrics, 149, 526—531
Publisher – Google Scholar
31.Healthcare Quality Improvement Partnership (2011). ‘National Diabetes Paediatric Audit Report 2009-2010,’http://www.hqip.org.uk/assets/NCAPOP-Library/NHSIC-National-Diabetes-Paediatric-Audit-Report-2009-2010.pdf.
Publisher
32.Helgeson, V. S., Becker, D., Escobar, O. & Siminerio, L. (2012). “Families with Children with Diabetes: Implications of Parent Stress for Parent and Child Health,” Journal of Pediatric Psychology, 37, 467—478.
Publisher – Google Scholar
33.Hershey, T., Perantie, D.C., Warren, S. L., Zimmerman, E. C., Sadler, M. & White, N. H. (2005). “Frequency and Timing of Severe Hypoglycemia Affects Spatial Memory in Children with Type 1 Diabetes,” Diabetes Care, 28, 2372-2377.
Publisher – Google Scholar
34.Ingerski, L. M., Anderson, B. J., Dolan, L. M. & Hood, K. K. (2010). “Blood Glucose Monitoring and Glycemic Control in Adolescence: Contribution of Diabetes-Specific Responsibility and Family Conflict,” Journal of Adolescent Health, 47, 191-197.
Publisher – Google Scholar
35.Jaser, S. S., Whittemore, R., Ambrosino, J., Lindemann, E. & Grey, M. (2008). “Mediators of Depressive Symptoms in Children with Type 1 Diabetes and Their Mothers,” Journal of Pediatric Psychology, 33, 509—519.
Publisher – Google Scholar
36.Jones, J. M., Lawson, M. L., Daneman, D., Olmsted, M. P. & Rodin, G. (2000). “Eating Disorders in Adolescent Females with and Without Type 1 Diabetes: Cross Sectional Study,” British Medical Journal, 320, 1563—1566.
Publisher – Google Scholar
37.Kokkonen, J., Taanila, A. & Kokkonen, E-R. (1997). “Diabetes in Adolescence: The Effect of Family and Psychologic Factors on Metabolic Control,” Nordic Journal of Psychiatry, 51, 165—172.
Publisher – Google Scholar
38.Kovacs, M., Goldston, D., Obrosky, D. S. & Bonar, L. K. (1997). “Psychiatric Disorders in Youth with IDDM: Rates and Risk Factors,” Diabetes Care, 20, 36—44.
Publisher – Google Scholar
39.Landolt, M. A., Ystrom, E., Sennhauser, F. H., Gnehm, H. E. & Vollrath, M. E. (2012). “The Mutual Prospective Influence of Child and Parental Post-Traumatic Stress Symptoms in Pediatric Patients,” Journal of Child Psychology and Psychiatry, 53, 767—774.
Publisher – Google Scholar
40.Lawrence, J. M., Standiford, D. A., Loots, B., Klingensmith, G. J., Williams, D. E., Ruggiero, A., Liese, A. D., Bell, R. A., Waitzfelder, B. E., McKeown, R. E. and The SEARCH for Diabetes in Youth Study. (2006). “Prevalence and Correlates of Depressed Mood among Youth with Diabetes: The SEARCH for Diabetes in Youth Study,” Pediatrics, 117, 1348—1358.
Publisher – Google Scholar
41.Liss, D. S., Waller, D. L., Kennard, B. D., McIntire, D., Capra, P. & Stephens, J. (1998). “Psychiatric Illness and Family Support In Children and Adolescents with Diabetic Ketoacidosis: A Controlled Study,” Journal of the American Academy of Child & Adolescent Psychiatry, 37, 536-544.
Publisher – Google Scholar
42.Luyckx, K., Seiffge-Krenke, I., Missotten, L., Rassart, J., Casteels, K. & Goethals, E. (2013). “Parent—adolescent Conflict, Treatment Adherence and Glycemic Control in Type 1 Diabetes: The Importance of Adolescent ExternalisingSymptoms,” Psychology & Health, Apr. 8, DOI:10.1080/08870446.2013.782405.
Publisher – Google Scholar
43.Lyoo, K., Yoon, S., Jacobson, A. M., Hwang, J., Musen, G., Kim, J. E., Simonson, D. C., Bae, S., Bolo, N., Kim, D. J., Weinger, K., Lee, J. H., Ryan, C. M. & Renshaw, P. F. (2012). “Prefrontal Cortical Deficits in Type 1 Diabetes Mellitus: Brain Correlates of Comorbid Depression,” Archives of General Psychiatry, 69, 1267-1276.
Publisher – Google Scholar
44.Mackey, E. R., Hilliard, M. E., Berger, S. S., Streisand, R. & Chen, R. (2011). “Individual and Family Strengths: An Examination of the Relation to Disease Management and Metabolic Control in Youth with Type 1 Diabetes,” Family Systems & Health, 29, 314-326.
Publisher – Google Scholar
45.Missotten, L. C., Luyckx, K. & Seiffge-Krenke, I. (2013). “Family Climate of Adolescents with and Without Type 1 Diabetes: Longitudinal Associations with Psychosocial Adaptation,” Journal of Child and Family Studies , 22, 344-354.
Publisher – Google Scholar
46.Monaghan, M., Singh, C., Streisand, R. & Cogen, F. R. (2010). “Screening and Identification of Children and Adolescents at Risk for Depression during a Diabetes Clinic Visit,” Diabetes Spectrum, 23, 25—31.
Publisher – Google Scholar
47.MTA Cooperative Group (1999). “A 14-month Randomized Clinical Trial of Treatment Strategies for Attention Deficit Hyperactivity Disorder,” Archives of General Psychiatry, 56, 1073 — 1086.
Publisher – Google Scholar
48.National Institute of Clinical Excellence, (2005). “Type 1 Diabetes: Diagnosis and Management of Type 1 Diabetes in Children, Young People And Adults,” Clinical Guideline 15 National Institute of Clinical Excellence, UK.http://www.nice.org.uk/cg15.
Publisher – Google Scholar
49.Northam, E. A., Matthews, L. K., Anderson, P. J., Cameron, F. J. & Werther, G. A. (2005). “Psychiatric Morbidity and Health Outcome in Type 1 Diabetes -Perspectives from a Prospective Longitudinal Study,” Diabetic Medicine, 22, 152-157.
Publisher – Google Scholar
50.Northam, E. A., Todd, S. & Cameron, F. J. (2006). “Interventions to Promote Optimal Health Outcomes in Children with Type 1 Diabetes — Are They Effective?” Diabetes Medicine, 23, 113-121.
Publisher – Google Scholar
51.Nylander, C., Toivonen, H., Nasic, S., Söderström, U., Tindberg, Y. & Fernell, E. (2013). “Children and Adolescents with Type 1 Diabetes and High Hba1c — A Neurodevelopmental Perspective,” Acta Pædiatrica, 102, 410—415.
Publisher – Google Scholar
52.Olson, D. H. & Gorall, D. M. (2003). “Circumplex Model of Marital and Family Systems,” In Normal Family Processes (3rd edn), F. Walsh (Ed), 514 — 547, Guilford Press, New York.
Publisher – Google Scholar
53.Olson, D. H., Gorall, D. M. & Tiesel, J. W. (2006). ‘FACES-IV Package: Administration. Life Innovations,’ Inc., Minneapolis, Minnesota.
54.Perantie, D. C., Koller, J. M., Weaver, P. M., Lugar, H. M., Black, K. J., White, N. H. & Hershey, T. (2011). “Prospectively Determined Impact of Type 1 Diabetes on Brain Volume during Development,” Diabetes, 60, 3006—3014.
Publisher – Google Scholar
55.Place, M., Martin, E., Hildreth, A. J., Wilson, J. & Hulsmeier, J. (1999). “Validating the Ontario Child Health Scale in a UK Population,” European Child and Adolescent Psychiatry, 8, 255-259.
Publisher – Google Scholar
56.Quittner, A., Espelage, D., Opipari, L., Carter, B., Eid, N. & Eigen, H. (1998). “Role Strain in Couples with and Without a Child with Chronic Illness: Associations with Marital Satisfaction, Intimacy, and Daily Mood,” Health Psychology, 17,112-124.
Publisher – Google Scholar
57.Rewers, A., Chase, H. P., Mackenzie, T., Walvarens, P., Roback, M. & Rewers, M. (2002). “Predictors of Acute Complications in Children with Type 1 Diabetes,” Journal of the American Medical Association, 287, 2511—2518.
Publisher – Google Scholar
58.Reynolds, K. A. & Helgerson, V. S. (2011). “Children with Diabetes Compared to Peers: Depressed? Distressed? A Meta-Analytic Review,” Annals of Behavioral Medicine. 42, 29-41
Publisher – Google Scholar
59.Robinson, R. C. & Price, T. R. (1982). “Post-stroke Depressive Disorders: A Follow-Up Study of 103 Patients,”Stroke, 13, 635-641.
Publisher – Google Scholar
60.Seiffge-Krenke, I. (1998). “The Highly Structured Climate in Families of Adolescents With Diabetes: Functional or Dysfunctional for Metabolic Control?,” Journal of Pediatric Psychology, 23, 313-322.
Publisher – Google Scholar
61.Silver, E., Westbrook, L. & Stein, R. (1998). “Relationship of Parental Psychological Distress to Consequences of Chronic Health Conditions in Children,” Journal of Pediatric Psychology, 23, 5-15.
Publisher – Google Scholar
62.Sinnamon, G. C. B., Caltabiano, M. & Baune, B. T. (2013). “Differentiating Disordered Affect in Children and Adolescents with Type 1 Diabetes,” Journal of Affective Disorders, 147, 51—58.
Publisher – Google Scholar
63.Skocić, M., Rudan, V., Brajković, L. & Marcinko, D. (2009). “Relationship among Psychopathological Dimensions, Coping Mechanisms, and Glycemic Control in a Croatian Sample of Adolescents with Diabetes Mellitus Type 1,”European Child & Adolescent Psychiatry, 19, 525-533.
Publisher – Google Scholar
64.Steele, M., Weiss, M., Swanson, J., Wang, J., Prinzo, R. S. & Binder, C. E. (2006). “A Randomized, Controlled Effectiveness Trial of OROS-Methylphenidate Compared to Usual Care with Immediate-Release Methylphenidate in Attention Deficit-Hyperactivity Disorder,” Canadian Journal of Clinical Pharmacology, 13, e50 — e62.
Publisher – Google Scholar
65.Streisand, R., Swift, E., Wickmark, T., Chen, R. & Holmes, C.S. (2005). “Pediatric Parenting Stress among Parents Of Children with Type 1 Diabetes: The Role of Self-Efficacy, Responsibility, and Fear,” Journal of Pediatric Psychology, 30, 513—521.
Publisher – Google Scholar
66.Swanson, J. M. (1992). ‘School-Based Assessments and Interventions for ADD Students,’ KC Publications, Irvine, California.
Google Scholar
67.Swanson, J., Gupta, S., Lam, A., Shoulson, I., Lerner, M., Modi, N., Lindemulder, E. & Wigal, S. (2003). “Development of a New Once-A-Day Formulation of Methylphenidate for the Treatment of Attention-Deficit/ Hyperactivity Disorder: Proof-Of-Concept and Proof-of-Product Studies,” Archives of General Psychiatry, 60, 204 — 211.
Publisher – Google Scholar
68.Whittemore, R., Urban, A. D., Tamborlane, W. V. & Grey, M. (2003). “Quality of Life in School-Age Children with Type 1 Diabetes on Intensive Treatment and Their Parents,” Diabetes Educator, 29, 847—854.
Publisher – Google Scholar
69.Wilkinson, P. (2013). “Non-suicidal Self-injury,” European Child & Adolescent Psychiatry, 22, 75-79.
Publisher – Google Scholar
70.Williams, L. B., Laffel,L. & Hood, K. K. (2009). “Diabetes-specific Family Conflict and Psychological Distress in Paediatric Type 1 Diabetes,” Diabetic Medicine, 26, 908—914.
Publisher – Google Scholar
71.Zashikhina, A. & Hagglof, B. (2009). “Family Functioning and Juvenile Chronic Physical Illness in Northern Russia,”Acta Paediatrica, 98, 355-360.
Publisher – Google Scholar









