Introduction
Cystic hygroma also known as water-tumor or lymphangioma is a benign malformation of lymphatic vessels commonly found in children but rarely in adults. It is the most frequently occurring type of lymphangiomas, comprising of single or multiple macrocystic lesions which can occur anywhere in the body. It usually occurs when the lymphatic system fails to communicate with the normal jugular vein which can occur in the head, neck, axilla, cervico-facial regions, groin and below the tongue. It can be due to genetic or environmental factors in children. In case of fetus, the cystic hygroma can develop into fetal hydrops that is, excess watery-fluid throughout the body resulting in fetal death whereas in some cases the baby survives however, develop other conditions like swelling, yellowish tan tumor and webbed neck. There is a possibility that the hygroma can be larger than the size of the fetus.
Cystic hygroma in children is usually associated with other chromosomal abnormalities like Turner syndrome (45 XO), Trisomy 13 (Patau syndrome), Trisomy 18 (Edward’s syndrome), Trisomy 21 (Down’s syndrome) and Noonan’s syndrome (45 X/ 46 XY). The environmental factors include maternal substance abuse such as consumption of alcohol and maternal viral infections such as one caused by Parvo virus of Fifth’s disease (Erythema infectiosum). Cystic hygroma is considered to be an indicator of heart malformations and congenital diaphragmatic hernia in children with normal karyotype as previously reported by Sananes et al. (2010) and Bulas et al. (1992). Schefter et al. (1985) and Antoniades et al. (2000) showed that upper respiratory tract infection or trauma could also trigger the onset of cystic hygroma in adults. Ali et al. (2006) reported that infection by Streptococcus or Staphylococcus species within the cyst can cause rapid enlargement of cystic mass resulting in airway obstruction. Sherman et al. (2001) and Suk et al. (1997) mentioned that less than 100 cases of adult cystic hygroma have been reported so far. In a research study, Karmody et al. (1982) showed that about 50-60% of the Lymphangiomas are congenital whereas 80-90% of tumor can be detected by 2 years of life.
Cystic hygroma is mainly characterized by the uncontrolled proliferation of the small lymphatic vessels with intervening fibrous tissue. It has been observed that some benign neoplasms have angiogenic activity that is generation of new capillaries from the pre-existing vessels. Maddalozzo et al. (1999) studies showed that the cells isolated from cystic hygroma tissue are angiogenic in nature. This angiogenic activity is due to the elevated levels of angiogenic inducer that is, basic fibroblast growth factor (bFGF) and low levels of angiogenic inhibitor that is, thrombospondin-1. Sauter et al. (1998) along with his co- worker showed upregulation of CD34 and CD31 and type VI collagen expression in Lymphangioma.
Leung et al. (1989) showed that VEGF (vascular endothelial growth factor) acts as a specific mitogen for vascular endothelial cells. According to D’Arcangelo et al. (2000), VEGF has the ability to induce vascular endothelial cell proliferation, cell migration and inhibits programmed cell death that is; apoptosis. Roberts et al. (1995) and Millauer et al. (1993) reported that VEGF plays a key role in regulation of angiogenesis and vasculogenesis. In a research study by Millauer et al. (1993), Kim et al. (1993) and Melnyk et al. (1996), it has been observed that deregulation of VEGF results in development of wide variety of solid tumors. In another study, Susanne N. et al. (2007) demonstrated elevated VEGFR-2 (vascular endothelial growth factor receptor) and R-3 and down-regulation of LYVE-1(lymphatic vessel endothelial hyaluronan receptor-1) in the lymphatic endothelial cells derived from lymphangioma tissue which helps to understand the etiology of lymphangiomas.
The discovery of these highly specific markers for lymphatic endothelial cells isolated from lymphangioma tissue has permitted the isolation and molecular characterization of markers other than endothelial markers in order to study the development of a cell line derived from cystic hygroma tumor. However, not much research has been carried out on the molecular marker expression on cystic hygroma tumor cells so as to define their mesenchymal and haematopoietic phenotypes along with pluripotency and cytokine properties. The main aim of our study is to develop a technology for isolation of cystic hygroma tumor cells and to understand the molecular mechanisms related to cystic hygroma tumor cells which are derived from cystic hygroma tumor tissue both at in vivo and in vitro levels. The other goal is to study the morphological features of cystic hygroma tumor cells in order to discern the origin of these cell types.
Materials and Methods
Establishment of Human Cystic Hygroma Cell Line
Cystic hygroma tumor tissue was removed by surgery and sent to Molecular Medicine laboratory under sterile conditions as per the ethical guidelines of Jaslok Hospital & Research Center. The tissue was cut into 2-4 mm pieces and washed 2-3 times in 1x PBS (Phosphate Buffer Saline, HiMedia, India) as per the protocol described by (Potdar and Chaugule 2011). In brief, the tissue pieces were digested with 0.25% Trypsin-EDTA (HiMedia, India) for 30 minutes at 37oC and cultured in 35 mm nunc petri dishes. The dishes were fed with freshly prepared growth medium DMEM (Dulbecco’s Modified Eagles’s Medium, HiMedia) supplemented with FBS (Fetal Bovine Serum, Invitrogen, Carlsbad, CA), Penicillin-Streptomycin, Insulin (Sigma, USA) and L-Glutamine (HiMedia). Cultures were then incubated in CO2 incubator at 37oC. The outgrowth of cells was monitored everyday under phase contrast microscope. Within 5-8 days, cells were found to be emerging out from explants. The area of similar cell type was marked and isolated using previously described puck cylinder method . These isolated cell type were then transferred to 35 mm tissue culture plates and were fed with freshly prepared growth medium (Puck T. et al 1956). Once the cells were confluent within 15-20 days, they were trypsinized with 0.25% Trypsin-EDTA and passaged. The same procedure was repeated after confluency and cells were further expanded in 50 mm tissue culture flask. These cells were used for RNA extraction and other experiments. Remaining cells were cryopreserved at -85oC till further use.
Phase Contrast Microscopy
Morphological analysis of cultured cells was performed using a phase contrast microscope (Carl Zeiss Co.) equipped with TSView software used for capturing images. The cells were observed under 20X and 40X magnifications. The cells were monitored regularly and images were captured for analysis of morphological features.
Light Microscopy
For the analysis using light microscopy, cystic hygroma cells were grown on the sterile coverslips and incubated for 2 days in growth media in order to attain partial confluence prior to Giemsa staining. Giemsa staining was performed in order to understand the basic morphological characteristics of the cystic hygroma cell line. Approximately 1×104 cells were grown on the sterile coverslips which were placed in 35mm dishes (Nunc). The cells were then fixed with 50% methanol for 10 minutes. Methanol was decanted and the cells were washed with 1X PBS. Cells were then treated with 3 ml filtered Giemsa stain (Fisher Scientific) followed by washing with distilled water to remove the excess of stain. The coverslip was air-dried and then observed under light microscope.
In Vitro Cytotoxicity Studies of Cystic Hygroma Stem Cell Line
The in vitro cytotoxicity assay was performed using MTT (3-(4, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay. In mitochondria, MTT is reduced to formazan crystals. This reaction was catalysed by the mitochondrial dehydrogenase enzyme. The wavelength at which formazan crystals absorb light is around 405nm which helps in measuring the viability of cells as well as rate of cell proliferation by monitoring the conversion of MTT into formazan crystals. The cystic hygroma cells were first counted using a Neubaur’s chamber. These cells were then seeded at a density of 1×104 cells per well in a 96-well plate. This experiment was performed in triplicates. Cells were fed with complete media and the media was changed every alternate day. After each time point, media was removed and replaced with 90 µl fresh growth media and 10 µl MTT (HiMedia, India) under dark conditions and incubated for 4 hours at 37°C in CO2 incubator. After 4 hours of incubation, media was removed and replaced with 200 µl dimethyl sulfoxide (DMSO, Merck). The absorbance was measured at 405 nm on microplate reader model SUNRISE (Tecan, India).
Characterization of Cystic Hygroma Stem Cell Line Using Different Molecular Markers
Total RNA was extracted from the cystic hygroma tissue for in vivo study and from the cystic hygroma cultured cells for in vitro study by using Trizol Reagent (Invitrogen, India). The isolated total RNA was quantified using spectrophotometer (Eppendorf, Germany). The cDNA was synthesized using Applied Biosystems High Capacity cDNA Kit (Applied Biosystem, Life Technologies Ltd.). Total 20 molecular markers were used to perform gene expression studies to characterize the cellular phenotype of cystic hygroma at in vivo and at in vitro level which include CD105, CD13, CD73, CD34, CD45, OCT4, NANOG, SOX2, LIF, KERATIN 18, CD44, cKIT, SSEA4, Ki67, EGFR, VIMENTIN, VEGF A, IL6, TNFα and β Actin as an internal control.
The molecular marker study was performed using Reverse Transcriptase PCR (RT/PCR). The PCR conditions and primer sequences for the respective genes have been reported in previous studies described by Potdar et al. (2010, 2011). The PCR products were analyzed for their respective amplifications on 2% Agarose gel and photographed using Gel Documentation system (Cell Biosciences, India).
Immunofluorescence
For immunofluorescence, the cells were directly seeded onto the sterile coverslips in 35mm culture dishes (Nunc). The confluent cells were fixed with 4% formaldehyde and incubated overnight. The culture coverslips were removed with the cell surface facing on top and placed in a clean and dry 35 mm dish. The coverslips were washed with 1 X PBS twice. The cells were then incubated with blocking buffer (1% BSA in 1X PBS) for 30 minutes at room temperature. Cells were incubated for 2 hours with primary antibodies EGFR (Mouse Anti-Human EGFR H11, DAKO Corporation, CA, USA), Ki67 (Purified AntiHumanKi67, BD Pharmingen TM, SanDiego CA) followed by secondary antibodies (FITC-labelled goat anti-mouse IgG) again for 2 hours at room temperature under dark conditions. After incubation, a drop of fluromount mounting media (Fluromount, Sigma, USA) was added on a clean grease-free slides and the culture coverslips were carefully placed on top of the mounting media. The edges of the coverslips were sealed using a quick dry nail-polish. After complete drying, the slides were observed under Carl Zeiss Phase Contrast Microscope using FITC filter for fluorescence microscopy.
Results
Morphological Analysis of Cystic Hygroma Cell Line
The cystic hygroma cells were isolated and cultured from the cystic hygroma tumor tissue as described above. Explant cultures were monitored regularly under phase contrast microscope. Outgrowth of cells was seen within 5 days of culture, from explant figure 1 (a). Figure 1(b) shows confluent cultures of cystic hygroma cell line having spindle shaped cells with distinct shiny nucleus and nuclei with scanty cytoplasmic granules giving it a clear appearance. These cells show mesenchymal and endothelial cell like appearance. Giemsa staining was performed to understand the general morphological characteristics and cytoplasmic structure of the cystic hygroma tumor cells. The stained cells show presence of more than one nucleus which are positioned at the centre of the cells as shown in the figure 1 (c). These cells showed high multiplication rate and few cells were found to be in the mitotic phase. Transformed cells showed presence of an aggregated cell clone as shown in the figure 1 (d).
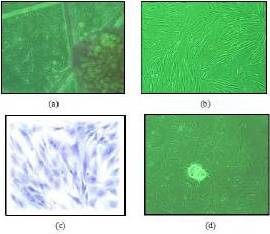
Figure 1: (a) Shows Phase Contrast Micrographs of Outgrowth of Cells from Cystic Hygroma Tumor Explant. (b) Shows Confluent Cultures of Cystic Hygroma Cell Line Having Spindle Shaped Cells Having Distinct, Round and Shiny Nucleus with Slight Cytoplasmic Granules Indicating Mesenchymal Cell Like Phenotype. (c) Represents the Light Microscope Images of Giemsa Stained Cystic Hygroma Tumor Cells which Show Presence of More than One Nucleus. (d) Shows Transformed Cells Forming an Aggregated Cell Clone.
In Vitro Cytotoxicity Studies of Cystic Hygroma Stem Cell Line
The cell growth study by MTT assay is used to estimate viability of cells and proliferation rate. The MTT assay plot (figure 2) shows an increase in cell proliferation (up to day 6). The formations of resultant purple formazan crystals were directly proportional to the energy metabolism in the cells. The doubling time of cystic hygroma tumor cells was found to be 38 hours.
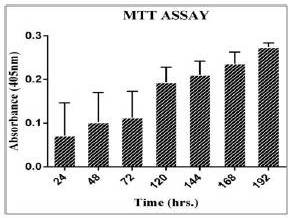
Figure 2: Shows a Bar Graph Indicating the Growth Rate of Cystic Hygroma Cell Line. The Standard Deviation for MTT Assay Readings were Taken from Day 1 to Day 6 was Plotted on the Graph Indicating a Steady Rise in Cell Proliferation Rate.
Characterization of Cystic Hygroma Tumor Tissue and Cystic Hygroma Cell Line Using Different Molecular Markers
The present study was undertaken to assess the molecular characterization of cystic hygroma tumor (in vivo) and cystic hygroma cell line (in vitro) in order to confirm specific phenotypes by using specific set of molecular markers such pluripotency, differentiation, mesenchymal, haematopoietic and cytokine markers.
Pluripotency and Differentiation Markers in Cystic Hygroma Tumor and Cystic Hygroma Cell Line
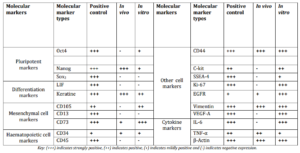
Expression of Oct4, Nanog, Sox2, LIF and Keratin 18 as markers for pluripotency and differentiation were studied in tumor tissue and in cystic hygroma cell line respectively as shown in the figure 3. It was observed that Oct4 and Sox2 were not expressed in vivo whereas, Nanog was up regulated. In case of in vitro, Oct4 and Nanog were mildly expressed whereas Sox2 was shown to be down regulated indicating that Sox2 plays some role in pathogenesis of cystic hygroma and needs further investigation. Differentiation markers LIF was down regulated both in vivo and in vitro whereas Keratin 18 was up regulated.
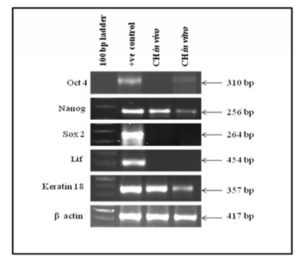
Figure 3: Shows the Expression of Pluripotency and Differentiation Cell Markers in Cystic Hygroma Tissue (in Vivo) and Cystic Hygroma Cell Line (in Vitro).
Mesenchymal and Haematopoietic cell Markers in Cystic Hygroma Tumor and Cystic Hygroma Cell Line
Mesenchymal and haematopoietic cell markers expression in cystic hygroma tumor (in vivo) and in cystic hygroma cell line(in vitro) is shown in figure 4. It was observed that CD105, CD13 and CD45 genes were not expressed in vivo. CD105 was found to be positive in vitro whereas, CD13 and CD45 were negative in vitro. CD73 and CD34 were found to be up regulated in both in vivo and in vitro indicating that they may be partially mesenchymal and haematopoietic in nature.
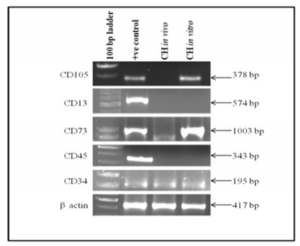
Figure 4: Shows Expression of Mesenchymal and Haematopoietic Cell Markers in Cystic Hygroma Tumor Tissue (in Vivo) and Cystic Hygroma Cell Line (in Vitro).
Other Cell Markers in Cystic Hygroma Tumor Tissue and in Cystic Hygroma Cell Line
It was observed that cell markers C-kit, SSEA4, Ki-67 and VEGF-A were expressed in vitro whereas CD44, EGFR and vimentin were expressed both in vivo and in vitro as shown in the figure 5.
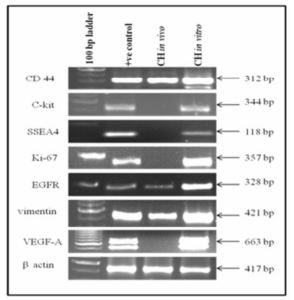
Figure 5: Show Expressions of Cell Markers in Cystic Hygroma Tumor Tissue (in Vivo) and Cystic Hygroma Cell Line (in Vitro).
Cytokine Markers in Cystic Hygroma Tumor Tissue and in Cystic Hygroma Cell Line
It was observed that cytokine markers IL-6 and TNF-α were expressed both in vivo and in vitro indicating that both IL-6 and TNF-α were regulators of angiogenesis. However, IL-6 was down regulated in vivo as shown in figure 6.
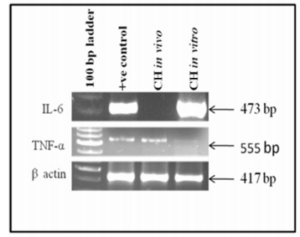
Figure 6: Shows Expression of Cytokine Markers in Cystic Hygroma Tumor Tissue and in Cystic Hygroma Cell Line.
Table 1: Shows Comparative Analysis of Cell Markers in Normal Placental Cell Line (Positive Control), Cystic Hygroma Tissue (in Vivo) and Cystic Hygroma Cells (in Vitro).
Immunofluorescence
Immunofluorescence is a simple and efficient technique to locate any specific cell types by using specific antibodies associated with these cells. In the present study, we have used 3 different primary antibodies (Ki-67, EGFR and Actin) in order to confirm their expression in the cystic hygroma cell line.
Localization of Ki-67, EGFR and Actin Proteins in Cystic Hygroma Cell Line
Ki-67 is a proliferative marker for tumor cells. It is a protein found only in the dividing cells and not in cells which are in the resting phase of the cell cycle. It is an antigen which induces immune system to produce antibodies against it. It has an important role in cell division. High level of Ki-67 is associated with poor prognosis of this disease. In the present study, it was observed that many cystic hygroma tumor cells expressed Ki-67 as shown in the figure 7 (a) and (b). Figure (a) shows the phase contrast microscopy of same cells without fluorescence.
In normal cells, EGFR, a surface protein receptor, plays an important role in cell growth and differentiation. However, aberrant activation of EGFR results in enhanced proliferation and tumorigenesis. It was seen that EGFR is expressed in many cystic hygroma cells as shown in the figure 7 (c) and (d). Figure (c) show the phase contrast microscopy of same cells without fluorescence.
β-Actin, a house-keeping gene, was used as an internal control in gene expression studies as this gene is expressed at constant levels in any pathological and non- pathological conditions which is shown in figure 7 (e) and (f). Figure (e) show the phase contrast microscopy of same cells without fluorescence.
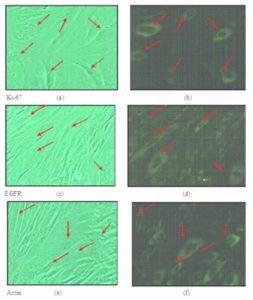
Figure 7: Shows Ki-67, EGFR and β-actin Protein Expression in Cystic Hygroma Cell Line: (b) Mild Expression of Ki-67 Protein Seen in Cystic Hygroma Cell Line. (d) High Expression of EGFR Protein Seen in Cystic Hygroma Cell Line. (f) High Expression of β-actin Protein Seen in Cystic Hygroma Cell Line. (a), (c) and (e) Phase Contrast Microscopy of Cystic Hygroma Cell Line without Fluorescence.
Discussion
Cystic hygroma is usually histologically characterized by proliferation of small lymphatic vessels with intervening fibrous tissue as described by Maddalozzo et al. (1999). The lymph sacs are developed at the mesoblast stage at the 6th week of embryonic life which is located in the neck between the subclavian and jugular veins. According to Russell et al. (2000) and Shahriari et al. (2001), it may either arise due to the segregation of the primitive embryonic lymphatic tissue or due to the congenital blocking of the regional lymph drainage. They are locally aggressive, benign lesions that are difficult to manage due to the recurrence of the tumor following surgery. Naresh et al. (2010) demonstrated that it is histologically characterized by a single layer endothelium which contains watery fluid i.e. lymph.
At the time of birth, the size of the tumor is small but enlarges gradually as it fills. Expansion of the hygroma may also occur due to internal bleeding or infection, due to which the overlying skin will have red or inflamed appearance. Cystic hygroma due to infection may be painful causing rapid enlargement of the cyst. This may reduce the antibiotic effect on the cyst due to which there is scarring in the cystic hygroma and in the surrounding tissue. This can lead to complications in the surgery as it becomes very difficult to control the infection. Therefore, there is a need to develop an in vitro model system of cystic hygroma tumor in order to study the biomarkers related to cystic hygroma which will be helpful in developing targeted therapies against hygroma tumor in young children.
In the present study, we have developed a technology to isolate cystic hygroma tumor cells from cystic hygroma tumor tissue and this cell line is at passage 11 and growing well in culture. These cells were grown in DMEM medium without EGF and designated as “Cystic Hygroma Cell line” (CHCL). The morphological features of cystic hygroma tumor cells shows spindle shaped cells with distinct shiny nucleus and nuclei with scanty cytoplasmic granules giving it a clear appearance. These cells show mesenchymal and endothelial cell like appearance. MTT assay shows that the doubling time of cystic hygroma tumor cells is very low indicating high proliferation rate.
Roberts et al. (1995) and Millauer et al. (1993) have earlier demonstrated that VEGF plays a very important role in regulation of angiogenesis and vasculogenesis and that deregulation of VEGF results in development of wide variety of solid tumors. Susanne et al. (2007) showed that elevation of VEGFR-2 (vascular endothelial growth factor receptor) and R-3 and down-regulation of LYVE-1 (lymphatic vessel endothelial hyaluronan receptor-1) in the lymphatic endothelial cells derived from lymphangioma tissue can helps in understanding the etiology of lymphangiomas. Nagy et al. (2002) studies demonstrated that VEP/ VEGF-A (vascular permeability factor/ vascular endothelial growth factor) act as a multifunctional cytokine which has an important role in pathological angiogenesis. In our study, VEGF-A is expressed at very high levelsin vitro indicating that they may be associated with pathological angiogenesis in cystic hygroma in young children. In a research study by Carson-Walter et al. (2001), Duff et al. (2003), Sullivan et al. (2003) and Fonsatti et al. (2004) demonstrated that CD105, a surface marker and TNF-α, a cytokine are found to be associated with lymphatic tumors. Our study correlates with these findings i.e.; CD105 is mildly expressed in vitro indicating that it can be used as a novel marker to characterize the differentiation status of mesenchymal cells in cystic hygroma tumor whereas, TNF-α is expressed bothin vivo and in vitro which may be a causative agent for cystic hygroma development in young children.
The recent discovery of these specific markers for lymphangiomas has permitted the isolation and molecular characterization of cystic hygroma tumor cells. However, the expression levels and the stability of different markers have not been studied so far which gives us an opportunity to study the origin of these tumor cells. In the present study, 4 different cell markers were used that is, pluripotent, mesenchymal, haematopoietic and cytokine markers. In case of pluripotency and differentiation markers, Nanog and Keratin 18 are expressed both in vivo and in vitro indicating that Nanog has the ability to maintain pluripotency in these tumor tissue as well as cells whereas, Keratin 18 helps maintain the cytoskeleton of these tissue and cells and may also indicate that the cystic hygroma tumor cells are of epithelial origin. Oct4 is mildly expressed in vitro but not in vivo indicating that Oct4 expression is linked to the undifferentiated phenotype.
In case of mesenchymal and haematopoietic cell markers, CD73 and CD34 is expressed both in vivo and in vitroindicating that CD73 shows partial mesenchymal phenotype in cystic hygroma tissue and cells whereas, CD34 shows haematopoietic phenotype and is acting as an endothelial marker which can be used to differentiate or identify the lymphatic malformations from the venous malformations especially in pathological practice in young children. In case of cytokine markers, IL-6 is expressed in vitro showing pro-inflammatory response in cystic hygroma cells.
Other cell markers which show association with cystic hygroma are CD44, C-kit, SSEA4, Ki-67, EGFR, vimentin and VEGF-A which are expressed in vitro. CD44 is usually associated with cell proliferation, cell differentiation, cell migration, and angiogenesis indicating that cystic hygroma cells may be showing one of these activities. C-kit is a tumor marker involved in cell migration and its expression in these tumor cells is a positive indicator for cell migration. SSEA-4 is a pluripotent embryonic marker which may associate with cystic hygroma tumor cells. Ki-67 is a proliferative marker expressed in these tumor cells which shows high proliferation rate. EGFR is a prognostic marker for most of the tumors and its expression in our study correlate with these findings. Vimentin expression is responsible for maintenance of cytoskeleton of cystic hygroma cells.
Immunofluorescence microscopy helps in localization of specific cell types. In the current study, we have selected 2 antibodies which were up-regulated in cystic hygroma cells in order to confirm the expression of EGFR and Ki-67 along with the β-Actin which acts as an internal control. In our study, it has been observed that EGFR and Ki-67 proteins were expressed in few specific cystic hygroma tumor cells. EGFR is highly expressed in most of the cancers and tumors. Normanno et al. (2006) reported that EGFR is expressed at high levels in vascular tumors indicating that EGFR plays an important role in vascular angiogenesis. Ki-67 is mildly expressed in cystic hygroma cells indicating that Ki-67 acts as a proliferative marker.
Overall, this paper describes the technology to develop cystic hygroma cell line from cystic hygroma tumor tissue. We are the first to report the development and characterization of this cell line by using several molecular markers which are expressed in this tumor. This will open up new avenues to understand the mechanism of progression of this disease in young children as well as to develop therapies for the treatment of cystic hygroma in the future.
Acknowledgements
We acknowledged with thanks the management of Jaslok Hospital and Research Center, Mumbai, India for sanctioning and supporting financial assistance. We are also thankful to Dr. A. R. Khan, OT Manager for supplying us cystic hygroma tumor tissue and Mrs. Naina Rane for technical help for this study.
References
Ali, G., Akif, A. & Faik, Ç. (2006). ‘Cystic Hygromas in Adults: Reports of Two Cases,’ Bak”ºrköy T”ºp Dergisi, 2 (3)101-103.
Google Scholar
Antoniades, K., Kiziridou, A. & Psimopoulou, M. (2000). “Traumatic Cervical Cystic Hygroma,” International Journal of Oral and Maxillofacial Surgery, 29 (1) 47—8.
Publisher – Google Scholar
Bulas, D. I., Saal, H. M., Allen, J. F., Kapur, S., Nies, B. M. & Newman, K. (1992). “Cystic Hygroma and Congenital Diaphragmatic Hernia: Early Prenatal Sonographic Evaluation of Fryns’ Syndrome,” Prenatal Diagnosis, 12 (11) 867-75.
Publisher – Google Scholar
Carson-Walter, E. B., Watkins, D. N., Nanda, A. et al. (2001). “Cell Surface Tumor Endothelial Markers are Conserved in Mice and Humans,” Cancer Research, 61 (18) 6649—55.
Publisher – Google Scholar
D’Arcangelo, D., Facchiano, F., Barlucchi, L. M., Melillo, G., Illi, B., Testolin, L., Gaetano, C. & Capogrossi, M. C. (2000). “Acidosis Inhibits Endothelial Cell Apoptosis and Function and Induces Basic Fibroblast Growth Factor and Vascular Endothelial Growth Factor Expression,” Circulation Research, 86, 312-318.
Publisher – Google Scholar
Duff, S. E., Li, C., Garland, J. M. et al. (2003). “CD105 is Important for Angiogenesis: Evidence and Potential Applications,” FASEB Journal,17 (9) 984—92.
Publisher – Google Scholar
Dvorak, H. F. (2002). “Vascular Permeability Factor/Vascular Endothelial Growth Factor: A Critical Cytokine in Tumor Angiogenesis and a Potential Target for Diagnosis and Therapy,” Journal of Clinical Oncology, 20 (21) 4368—80.
Publisher – Google Scholar
Fonsatti, E. & Maio, M. (2004). “Highlights on endoglin (CD105): From Basic Findings towards Clinical Applications in Human Cancer,” Journal of Translational Medicine, 2 (1)18.
Publisher – Google Scholar
Karmody, C. S., Fortson, J. K. & Calcaterra, V. E. (1982). “Lymphangiomas of the Head and Neck in Adults,”Otolaryngology- Head and Neck Surgery, 90 (3 Pt 1) 283-8.
Publisher – Google Scholar
Kim, K. J., Li, B., Winer, J., Armanini, M., Gillett, N., Phillips, H. S. & Ferrara, N. (1993). “Inhibition of Vascular Endothelial Growth Factor-Induced Angiogenesis Suppresses Tumour Growth in Vivo,” Nature, 362 (6423) 841-4.
Publisher – Google Scholar
Kumar, N., Kohli, M., Pandey, S. & Tulsi, S. P. S. (2010). “Cystic Hygroma,” National Journal of Maxillofacial Surgery, 1(1) 81—85.
Publisher – Google Scholar
Leung, D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V. & Ferrara, N. (1989). “Vascular Endothelial Growth Factor is a Secreted Angiogenic Mitogen,” Science, 246 (4935)1306-9.
Publisher – Google Scholar
Maddalozzo, J., Hughes, C. A., Huang, L., Mu, Y., Ludemann, J. & Crawford, S. (1999). “High Angiogenic Activity in Cells Isolated from Cystic Hygroma: Role of bFGF,” Archives Otolaryngology- Head and Neck Surgery, 125 (1) 45-8.
Publisher – Google Scholar
Melnyk, O., Shuman, M. A. & Kim, K. J. (1996). “Vascular Endothelial Growth Factor Promotes Tumor Dissemination by a Mechanism Distinct from its Effect on Primary Tumor Growth,” Cancer Research, 56 (4) 921-4.
Publisher – Google Scholar
Millauer, B., Wizigmann-Voos, S., Schnurch, H., Martinez, R., Moller, N. P., Risau, W. & Ullrich, A. (1993). “High Affinity VEGF Binding and Developmental Expression Suggest Flk-1 as a Major Regulator of Vasculogenesis and Angiogenesis,”Cell, 72 (6) 835-46.
Publisher – Google Scholar
Nagy, J. A., Vasile, E., Feng, D., Sundberg, C., Brown, L. F., Detmar, M. J., Lawitts, J. A., Benjamin, L., Tan, X., Manseau, E. J., Dvorak, A. M. & Dvorak, H. F. (2002). “Vascular Permeability Factor/Vascular Endothelial Growth Factor Induces Lymphangiogenesis as Well as Angiogenesis,” Journal of Experimental Medicine, 196 (11) 1497-506.
Publisher – Google Scholar
Norgall, S., Papoutsi, M., Rossler, J., Schweigerer, L., Wilting, J. & Weich, H. A. (2007). “Elevated Expression of VEGFR-3 in Lymphatic Endothelial Cells from Lymphangiomas,” BioMed Central Cancer, 7 (1) 105.
Publisher – Google Scholar
Normanno, N., De Luca, A., Bianco, C., Strizzi, L., Mancino, M., Maiello, M. R., Carotenuto, A., De Feo, G., Caponigro, F. & Salomon, D. S. (2006). “Epidermal Growth Factor Receptor (EGFR) Signaling in Cancer,” Gene, 366 (1) 2-16.
Publisher – Google Scholar
Potdar, P. & Chaugule, S. (2011). “Establishment and Molecular Characterization of Breast Cancer Mesenchymal Stem Cell Line Derived from Human Non-Metastasis Breast Cancer Tumor,” Stem Cell Discovery, 1 (2) 21—28.
Publisher – Google Scholar
Potdar, P. D. & Sutar, J. P. (2010). “Establishment and Molecular Characterization of Mesenchymal Stem Cell Lines Derived from human Visceral & Subcutaneous Adipose Tissues,” Journal of Stem cell and Regenerative Medicine, 6 (1) 26—35.
Publisher – Google Scholar
Puck, T. T., Marcus, P. I. & Cieciura, S. J. (1956). “Clonal Growth of Mammalian Cells in Vitro,” Journal of Experimental Medicine,103 (2) 273-284.
Publisher – Google Scholar
Roberts, W. G. & Palade, G. E. (1995). “Increased Microvascular Permeability and Endothelial Fenestration Induced by Vascular Endothelial Growth Factor,” Journal Cell Science, 108 (Pt 6) 2369-79.
Publisher – Google Scholar
Salomäki, H. H., Sainio, A. O., Söderström, M., Pakkanen, S., Laine, J. & Järveläinen, H. T. (2008). “Differential Expression of Decorin by Human Malignant and Benign Vascular Tumors,” Journal of Histochemistry & Cytochemistry, 56 (7) 639—646.
Publisher – Google Scholar
Sananes, N., Guigue, V., Kohler, M., Bouffet, N., Cancellier, M., Hornecker, F., Hunsinger, M. C., Kohler, A., Mager, C., Neumann, M., Schmerber, E., Tanghe, M., Nisand, I. & Favre, R. (2010). “Nuchal Translucency and Cystic Hygroma Colli in Screening for Fetal Major Congenital Heart Defects in a Series of 12,910 Euploid Pregnancies,” Ultrasound in Obstetrics & Gynecology, 35 (3) 273-9.
Publisher – Google Scholar
Sauter, B., Foedinger, D., Sterniczky, B., Wolff, K. & Rappersberger, K. (1998). “Immunoelectron Microscopic Characterization of Human Dermal Lymphatic Microvascular Endothelial Cells. Differential Expression of CD31, CD34, and Type IV Collagen with Lymphatic Endothelial Cells vs Blood Capillary Endothelial Cells in Normal Human Skin, Lymphangioma, and Hemangioma in Situ,” Journal of Histochemistry & Cytochemistry, 46 (2) 165-76.
Publisher – Google Scholar
Schefter, R. P., Olsen, K. D. & Gaffey, T. A. (1985). “Cervical Lymphangioma in the Adult,” Otolaryngology- Head and Neck Surgery, 93 (1) 65—69.
Publisher – Google Scholar
Shahriari, A. & Odell, J. A. (2001). “Cervical and Thoracic Components of Multiorgan Lymphangiomatosis Managed Surgically,” The Annals of Thoracic Surgery,71 (2) 694—6.
Publisher – Google Scholar
Sherman, B. E. & Kendall, K. (2001). “A Unique Case of the Rapid Onset of a Large Cystic Hygroma in the Adult,”American Journal of Otolaryngology, 22 (3) 206-210.
Publisher – Google Scholar
Suk, S., Sheridan, M. & Saenger, J. S. (1997). “Adult Lymphangioma: A Case Report,” Ear Nose & Throat Journal, 76 (12) 881-883.
Publisher – Google Scholar
Sullivan, D. C., Huminiecki, L., Moore, J. W. et al. (2003). “EndoPDI, a Novel Protein-Disulfide Isomerase-Like Protein that is Preferentially Expressed in Endothelial Cells Acts as a Stress Survival Factor,” The Journal of Biological Chemistry, 278 (47) 47079—88.
Publisher – Google Scholar
Williams, N. S., Bulstrode, C. J. K. & Russell, R. C. (2000). Bailey and Love’s Short Practice of Surgery, Arnold, editor.London and USA Oxford University Press.
Publisher – Google Scholar











