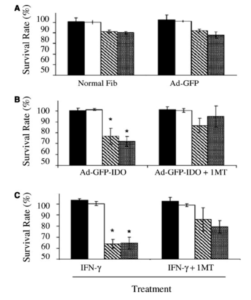
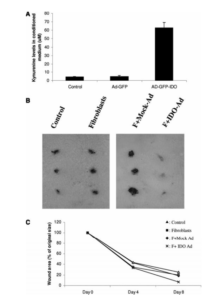
Aubock, J., Irschick, E., Romani, N., Kompatscher, P., Hopfl, R., Herold, M., Schuler, G., Bauer, M., Huber, C. & Fritsch, P. (1988). “Rejection after a Slightly Prolonged Survival Time of Langerhans Cell-Depleted Allogeneic Cultured Epidermis Used for Wound Coverage in Humans,” Transplantation, 45:730.
Publisher – Google Scholar
Bahar, M. A., Nabai, L. & Ghahary, A. (2012). “Immunoprotective Role of Indoleamine 2,3-Dioxygenase in Engraftment of Allogeneic Skin Substitute in Wound Healing,” Journal of Burn Care & Research, 33: 364-70.
Publisher – Google Scholar
Ball, H. J., Sanchez-Perez, A., Weiser, S., Austin, C. J., Astelbauer, F., Miu, J., McQuillan, J. A., Stocker, R., Jermiin, L. S. & Hunt, N. H. (2007)0 “Characterization of an Indoleamine 2,3-Dioxygenase-Like Protein Found in Humans and Mice,”Gene, 396: 203-13.
Publisher – Google Scholar
Beutelspacher, S. C., Tan, P. H., McClure, M. O., Larkin, D. F., Lechler, R. I. & Geory, A. J. (2006). “Expression of Indoleamine 2,3-Dioxygenase (IDO) by Endothelial Cells: Implications for the Control of Alloresponses,” American Journal of Transplantation, 6(6): 1320-30.
Publisher – Google Scholar
Brandacher, G., Perathoner, A., Ladurner, R., Schneeberger, S., Obrist, P., Winkler, C. et al. (2006). “Prognostic Value of Indoleamine 2,3-Dioxygenase Expression in Colorectal Cancer: Effect on Tumor-Infiltrating T Cells,” Clinical Cancer Research, 12: 1144—51.
Publisher – Google Scholar
Brauner, R., Nonoyama, M., Laks, H., Drinkwater, Jr D. C., McCaffery, S., Drake, T., Berk, A. J., Sen, L. & Wu, L. (1997). “Intracoronary Adenovirus-Mediated Transfer of Immunosuppressive Cytokine Gene Prolongs Allograft Survival,” The Journal of Thoracic and Cardiovascular Surgery, 114: 923.
Publisher – Google Scholar
Burkin, D. J., Kimbro, K. S., Barr, B. L., Jones, C., Taylor, M. W. & Gupta, S. L. (1993). “Localization of the Human Indoleamine 2,3-Dioxygenase (IDO) Gene to the Pericentromeric Region of Human Chromosome 8,” Genomics, 17(1): 262-3.
Publisher – Google Scholar
Chaves, A. C., Ceravolo, I. P., Gomes, J. A., Zani, C. L., Romanha, A. J. & Gazzinell, R. T. (2001). “IL-4 and IL-13 Regulate the Induction of Indoleamine 2,3-Dioxygenase Activity and the Control of Toxoplasma Gondii Replication in Human Fibroblasts Activated with IFN-Gamma,” European Journal of Immunology, 31(2): 333-44.
Publisher – Google Scholar
Chavez-Munoz, C., Hartwell, R., Jalili, R. B., Carr, M., Kilani, R. T., Jafarnejad, S. M., Rahmani-Neishabour, E., Forouzandeh, F., Boyce, S. T. & Ghahary, A. (2012). “Application of an Indoleamine 2,3-Dioxygenase-Expressing Skin Substitute Improves Scar Formation in a Fibrotic Animal Model,” Journal of Investigative Dermatology, 132: 1501-05.
Publisher – Google Scholar
Ciorba, M. A. (2013). “Indoleamine 2,3 Dioxygenase in Intestinal Disease,” Current Opinion in Gastroenterology, 29(2): 146-52.
Publisher – Google Scholar
de Jong, R. A., Kema, I. P., Boerma, A., Boezen, H. M., der Want, J. J., Gooden, M. J., Hollema, H. & Nijman, H. W. (2012). “Prognostic Role of Indoleamine 2,3-Dioxygenase in Endometrial Carcinoma,” Gynecological Oncology, 26(3):474-80.
Publisher – Google Scholar
Drachenberg, C. B., Klassen, D. K., Weir, M. R., Wiland, A., Fink, J. C., Bartlett, S. T., Cangro, C. B., Blahut, S. & Papadimitriou, J. C. (1999). “Islet Cell Damage Associated with Tacrolimus and Cyclosporine: Morphological Features in Pancreas Allograft Biopsies and Clinical Correlation,” Transplantation, 68: 396—402.
Publisher – Google Scholar
Fallarino, F., Grohmann, U., Vacca, C., Bianchi, R., Orabona, C., Spreca, A., Fioretti, M. C. & Puccetti, P. (2002). “T Cell Apoptosis by Tryptophan Catabolism,” Cell Death & Differentiation, 9: 1069—77.
Publisher – Google Scholar
Fallarino, F., Grohmann, U., You, S., McGrath, B. C., Cavener, D. R., Vacca, C., Orabona, C., Bianchi, R., Belladonna, M. L., Volpi, C., Santamaria, P., Fioretti, M. C. & Puccetti, P. (2006). “The Combined Effects of Tryptophan Starvation and Tryptophan Catabolites Down-Regulate T Cell Receptor Zeta-Chain and Induce a Regulatory Phenotype in Naive T Cells,”The Journal of Immunology, 176: 6752—61.
Publisher – Google Scholar
Fiorina, P. & Secchi, A. (2007). “Pancreatic Islet Cell Transplant for Treatment of Diabetes,” Endocrinology and Metabolism Clinics of North America, 36: 999—1013.
Publisher – Google Scholar
Forouzandeh, F., Jalili, R. B., Germain, M., Duronio, V. & Ghahary, A. (2008a). “Skin Cells, but not T Cells, are Resistant to Indoleamine 2, 3-Dioxygenase (IDO) Expressed by Allogeneic Fibroblasts,” Wound Repair and Regeneration, 16: 379—87.
Publisher – Google Scholar
Forouzandeh, F., Jalili, R. B., Germain, M., Duronio, V. & Ghahary, A. (2008b). “Differential Immunosuppressive Effect of Indoleamine 2,3-Dioxygenase (IDO) on Primary Human CD4 and CD8 T Cells,” Molecular and Cellular Biochemistry, 309: 1—7.
Publisher – Google Scholar
Froud, T., Baidal, D. A., Ponte, G., Ferreira, J. V., Ricordi, C. & Alejandro, R. (2006). “Resolution of Neurotoxicity and β-Cell Toxicity in an Islet Transplant Recipient Following Substitution of Tacrolimus with MMF,” Cell Transplantation, 15: 613—20.
Publisher – Google Scholar
Frumento, G., Rotondo, R., Tonetti, M., Damonte, G., Benatti, U. & Ferrara, G. B. (2002). “Tryptophan-Derived Catabolites are Responsible for Inhibition of T and Natural Killer Cell Proliferation Induced by Indoleamine 2,3-Dioxygenase,” The Journal of Experimental Medicine, 196: 459—68.
Publisher – Google Scholar
Furset, G., Floisand, Y. & Sioud, M. (2008). “Impaired Expression of Indoleamine 2, 3-Dioxygenase in Monocyte-Derived Dendritic Cells in Response to Toll-Like Receptor-7/8 Ligands,” Immunology, 123: 263—71.
Publisher – Google Scholar
Gallico, G. G., O’ Connor, N. E., Compton, C. C., Kehinde, O. & Green, H. (1984). “Permanent Coverage of Large Burn Wound with Autologous Cultured Human Epithelium,” The New England Journal of Medicine, 311:448.
Publisher – Google Scholar
Ghahary, A., Li, Y., Tredget, E. E. et al. (2004). “Expression of Indoleamine 2,3-Dioxygenase in Dermal Fibroblasts Functions as a Local Immunosuppressive Factor,” Journal of Investigative Dermatology, 122: 953—64.
Publisher – Google Scholar
Green, H., Kehinde, O. & Thomas, J. (1993). “Growth of Cultured Human Epidermal Cells into Multiple Epithelia Suitable for Grafting,” Proceedings of the National Academy of Sciences of the United States of America, 76:5665.
Publisher – Google Scholar
Grohmann, U., Fallaraino, F. & Puccetti, P. (2003). “Tolerance, DCs and Tryptophan: Much Ado about IDO,” Trends in Immunology, 24(5): 242-8.
Publisher – Google Scholar
Grohmann, U., Volpi, C., Fallarino, F., Bozza, S., Bianchi, R., Vacca, C., Orabona, C., Belladonna, M. L., Ayroldi, E., Nocentini, G., Boon, L., Bistoni, F., Fioretti, M. C., Romani, L., Riccardi, C. & Puccetti, P. (2007). “Reverse Signaling through GITR Ligand Enables Dexamethasone to Activate IDO in Allergy,” Nature Medicine, 13: 579—86.
Publisher – Google Scholar
Guillemin, G. J., Brew, B. J., Noonan, C. E., Takikawa, O. & Cullen, K. M. (2005) “Indoleamine 2,3-Dioxygenase and Quinolinic Acidimmunoreactivity in Alzheimer’s Disease Hippocampus,” Neuropathology and Applied Neurobiology, 31: 395—404.
Publisher – Google Scholar
Hansen, A. M., Driussi, C., Turner, V., Takikawa, O. & Hunt, N. H. (2000). “Tissue Distribution of Indoleamine 2,3-Dioxygenase in Normal and Malaria-Infected Tissue,” Redox Report, 5:112.
Publisher – Google Scholar
Hayaishi, O. (1996). “Utilization of Superoxide Anion by Indoleamine Oxygenase-Catalyzed Tryptophan and Indoleamine Oxidation,” Advances in Experimental Medicine and Biology, 398: 285—289.
Publisher – Google Scholar
Hayashi, T., Beck, L., Rossetto, C., Gong, X., Takikawa, O., Takabayashi, K. et al. (2004). “Inhibition of Experimental Asthma by Indoleamine 2,3-Dioxygenase,” The Journal of Clinical Investigation, 114(2): 270-9.
Publisher – Google Scholar
Heyes, M. P., Saito, K., Crowley, J. S., Davis, L. E., Demitrack, M. A., Der, M., Dilling, L. A., Elia, J., Kruesi, M. J. P., Lackner, A., Larsen, S. A., Lee, K., Leonard, H. L., Markey, S. P., Martin, A., Milstein, S., Mouradian, M. M., Pranzatelli, M. R., Quearry, B. J., Salazar, A., Smith, M., Strauss, S. E., Sunderland, T., Swedo, S. W. & Tourtellotte, W. W. (1992). “Quinolinic acid and Kynurenine Pathway Metabolism in Inflammatory and Non-Inflammatory Neurological Disease,”Brain, 115: 1249—73.
Publisher – Google Scholar
Hirata, F. & Hayaishi, O. (1975). “Studies on Indoleamine 2,3-Dioxygenase. I. Superoxide Anion as Substrate,” The Journal of Biological Chemistry, 250: 5960—6.
Publisher – Google Scholar
Hou, D. Y., Muller, A. J., Sharma, M. D., DuHadaway, J., Banerjee, T., Johnson, M. et al. (2007). “Inhibition of Indoleamine 2,3-Dioxygenase in Dendritic Cells by Stereoisomers of 1-Methyl-Tryptophan Correlates with Antitumor Responses,”Cancer Research, 67: 792—801.
Publisher – Google Scholar
Hwu, P., Du, M. X., Lapointe, R., Do, M., Taylor, M. W. & Young, H. A. (2000). “Indoleamine 2,3-Dioxygenase Production by Human Dendritic Cells Results in the Inhibition of T Cell Proliferation,” The Journal of Immunology, 164: 3596—9.
Publisher – Google Scholar
Inaba, T., Ino, K., Kajiyama, H., Shibata, K., Yamamoto, E., Kondo, S. et al. (2010). “Indoleamine 2,3-Dioxygenase Expression Predicts Impaired Survival of Invasive Cervical Cancer Patients Treated with Radical Hysterectomy,”Gynecologic Oncology, 117: 423—8.
Publisher – Google Scholar
Inaba, T., Ino, K., Kajiyama, H., Yamamoto, E., Shibata, K., Nawa, A. et al. (2009). “Role of the Immunosuppressive Enzyme Indoleamine 2,3-Dioxygenase in the Progression of Ovarian Carcinoma,” Gynecologic Oncology, 115: 185—92.
Publisher – Google Scholar
Ino, K., Yamamoto, E., Shibata, K., Kajiyama, H., Yoshida, N., Terauchi, M. et al. (2008). “Inverse Correlation between Tumoral Indoleamine 2,3-Dioxygenase Expression and Tumor-Infiltrating Lymphocytes in Endometrial Cancer: Its Association with Disease Progression and Survival,” Clinical Cancer Research, 14: 2310—17.
Publisher – Google Scholar
Jalili, R. B., Forouzandeh, F., Ali Bahar, M. & Ghahary, A. (2007). “The Immunoregulatory Function of Indoleamine 2,3 Dioxygenase and its Application in Allotransplantation,” Iranian Journal of Allergy, Asthma and Immunology, 6(4): 167-79.
Publisher – Google Scholar
Jalili, R. B., Forouzandeh, F., Rezakhanlou, A. M., Hartwell, R., Medina, A., Warnock, G. L., Larijani, B. & Ghahary, A. (2010). “Local Expression of Indoleamine 2,3-Dioxygenase in Syngeneic Fibroblasts Significantly Prolongs Survival of an Engineered Three-Dimensional Islet Allograft,” Diabetes, 59: 2219-27.
Publisher – Google Scholar
Johnson, B. A., Baban, B. & Mellor, A. L. (2009). “Targeting the Immunoregulatory Indoleamine 2,3 Dioxygenase Pathway in Immunotherapy,” Immunotherapy, 1 (4): 645-61.
Publisher – Google Scholar
Kyewski, B. & Klein, L. (2006). “A Central Role for Central Tolerance,” Annual Review of Immunology, 24: 571—606.
Publisher – Google Scholar
Li, X. K., Okuyama, T., Tamura, A., Enosawa, S., Kaneda, Y., Takahara, S., Funashima, N., Yamada, M., Amemiya, H. & Suzuki, S. (1998). “Prolonged Survival of Rat Liver Allografts Transfected with Fas Ligand-Expressing Plasmid1,”Transplantation, 66: 1416.
Publisher – Google Scholar
Li, Y., Tredget, E. E., Ghaffari, A., Lin, X., Kilani, R. T. & Ghahary, A. (2006). “Local Expression of Indoleamine 2,3-Dioxygenase Protects Engraftment of Xenogeneic Skin Substitute,” Journal of Investigative Dermatology, 126: 128—36.
Publisher – Google Scholar
Li, Y., Tredget, E. E. & Ghahary, A. (2004). “Cell Surface Expression of MHC Class I Antigen is Suppressed in Indoleamine 2,3-Dioxygenase Genetically Modified Keratinocytes: Implications in Allogeneic Skin Substitute Engraftment,”Human Immunology, 65(2): 114-23.
Publisher – Google Scholar
Lob, S. & Konigsrainer, A. (2009). “Role of IDO in Organ Transplantation: Promises and Difficulties,” International Reviews of Immunology, 28(3-4): 185-206.
Publisher – Google Scholar
Mahanonda, R., Sa-Ard-Iam, N., Montreekachon, P., Pimkhaokham, A., Yongvanichit, K., Fukuda, M. M. & Pichyangkul, S. (2007). “IL-8 and IDO Expression by Human Gingival Fibroblasts via TLRs,” The Journal of Immunology, 178: 1151—57.
Publisher – Google Scholar
Malina, H. Z. & Martin, X. D. (1993). “Indoleamine 2,3-Dioxygenase Activity in the Aqueous Humor, Iris/Ciliary Body, and Retina of the Bovine Eye,” Graefe’s Archive for Clinical and Experimental Ophthalmology, 231: 482.
Publisher – Google Scholar
Mellor, A. L., Keskin, D. B., Johnson, T., Chandler, P. & Munn, D. H. (2002). “Cells Expressing Indoleamine 2,3-Dioxygenase Inhibit T Cell Responses,” The Journal of Immunology, 168: 3771—6.
Publisher – Google Scholar
Mellor, A. L. & Munn, D. H. (1999). “Tryptophan Catabolism and T-Cell Tolerance: Immunosuppression by Starvation,”Immunology Today, 20: 469—73.
Publisher – Google Scholar
Mellor, A. L. & Munn, D. H. (2004). “IDO Expression by Dendritic Cells: Tolerance and Tryptophan Catabolism,” Nature Reviews Immunology, 4(10): 762-74.
Publisher – Google Scholar
Muller, A. J. & Prendergast, G. C. (2007) “Indoleamine 2,3-Dioxygenase in Immune Suppression and Cancer,” Current Cancer Drug Targets, 7(1): 31-40.
Publisher – Google Scholar
Munn, D. H. & Mellor, A. L. (2007). “Indoleamine 2,3-Dioxygenase and Tumour-Induced Tolerance,” The Journal of Clinical Investigation, 117(5): 1147-54.
Publisher – Google Scholar
Munn, D. H., Shafizadeh, E., Attwood, J. T., Bondarev, I., Pashine, A. & Mellor, A. L. (1999). “Inhibition of T Cell Proliferation by Macrophage Tryptophan Catabolism,” The Journal of Experimental Medicine, 189: 1363—72.
Publisher – Google Scholar
Munn, D. H., Sharma, M. D., Hou, D., Baban, B., Lee, J. R., Antonia, S. J., Messina, J. L., Chandler, P., Koni, P. A. & Mellor, A. L. (2004). “Expression of Indoleamine 2,3-Dioxygenase by Plasmacytoid Dendritic Cells in Tumor-Draining Lymph Nodes,” The Journal of Clinical Investigation, 114: 280—90.
Publisher – Google Scholar
Munn, D. H., Zhou, M., Attwood, J. T., Bondarev, I., Conway, S. J., Marshall, B. et al. (1998). “Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism,” Science, 281(5380):1191-3.
Publisher – Google Scholar
Musso, T., Gusella, G. L., Brooks, A., Longo, D. L. & Varesio, L. (1994). “Interleukin-4 Inhibits Indoleamine 2,3-Dioxygenase Expression in Human Monocytes,” Blood, 83(5): 1408-11.
Publisher – Google Scholar
Odemuyiwa, S. O., Ghahary, A., Li, Y., Puttagunta, L., Lee, J. E., Musat-Marcu, S. et al. (2004). “Cutting Edge: Human Eosinophils Regulate T Cell Subset Selection through Indoleamine 2,3-Dioxygenase,” The Journal of Immunology, 173(10): 5909-13.
Publisher – Google Scholar
Pan, K., Wang, H., Chen, M. S., Zhang, H. K., Weng, D. S., Zhou, J. et al. (2008). “Expression and Prognosis Role of Indoleamine 2,3-Dioxygenase in Hepatocellular Carcinoma,” Journal of Cancer Research and Clinical Oncology, 134: 1247—53.
Publisher – Google Scholar
Phillips, T. J. (1991). “Cultured Epidermal Allografts-a Permanent or Temporary Solution?,” Transplantation, 51: 937.
Publisher – Google Scholar
Qian, F., Villella, J., Wallace, P. K., Mhawech-Fauceglia, P., Tario, Jr J. D., Andrews, C. et al. (2009). “Efficacy of Levo-1-Methyl Tryptophan and Dextro-1-Methyl Tryptophan in Reversing Indoleamine-2,3-Dioxygenase-Mediated Arrest of T-Cell Proliferation in Human Epithelial Ovarian Cancer,” Cancer Res, 69: 5498—504.
Publisher – Google Scholar
Raju, G. P., Belland, S. E. & Eisen, H. J. (1994). “Prolongation of Cardiac Allograft Survival with Transforming Growth Factor-Beta 1 in Rats,” Transplantation, 58: 392.
Publisher – Google Scholar
Reddy, P., Sun, Y., Toubai, T., Duran-Struuck, R., Clouthier, S. G., Weisiger, E., Maeda, Y., Tawara, I., Krijanovski, O., Gatza, E., Liu, C., Malter, C., Mascagni, P., Dinarello, C. A. & Ferrara, J. L. (2008). “Histone Deacetylase Inhibition Modulates Indoleamine 2,3-Dioxygenase-Dependent DC Functions and Regulates Experimental Graft-versus-Host Disease in Mice,” The Journal of Clinical Investigation, 118: 2562—73.
Publisher – Google Scholar
Reddy, P., Sun, Y., Toubai, T., Duran-Struuck, R., Clouthier, S. G., Weisiger, E., Maeda, Y., Tawara, I., Krijanovski, O., Gatza, E., Liu, C., Malter, C., Ricordi, C., Hering, B. J. & Shapiro, A. M. (2008). ‘The Clinical Islet Transplantation Consortium. Î’ Cell Transplantation for Diabetes Therapy,’ Lancet, 372: 27—28.
Riesenberg, R., Weiler, C., Spring, O., Eder, M., Buchner, A., Popp, T. et al. (2007). “Expression of Indoleamine 2,3-Dioxygenase in Tumor Endothelial Cells Correlates with Long-Term Survival of Patients with Renal Cell Carcinoma,” Clin Cancer Res, 13: 6993—7002.
Publisher – Google Scholar
Rouabhia, M., Germain, L., Belanger, F. & Auger, F. A. (1993). “Cultured Epithelium Allografts: Langerhans Cell and Thy-1 Dendritic Epidermal Cell Depletion Effects on Allograft Rejection,” Transplantation, 56: 259.
Publisher – Google Scholar
Sardar, A. M. & Reynolds, G. P. (1995). “Frontal Cortex Indoleamine-2,3-Dioxygenase Activity is Increased in HIV-1-Associated Dementia,” Neuroscience Letters, 187: 9—12.
Publisher – Google Scholar
Sharma, M. D., Baban, B., Chandler, P., Hou, D. Y., Singh, N., Yagita, H., Azuma, M., Blazar, B. R., Mellor, A. L. & Munn, D. H. (2007). “Plasmacytoid Dendritic Cells from Mouse Tumor-Draining Lymph Nodes Directly Activate Mature Tregs via Indoleamine 2,3-Dioxygenase,” The Journal of Clinical Investigation, 117: 2570—582.
Publisher – Google Scholar
Soliman, H., Varela, M. M. & Antonia, S. (2010). “Indoleamine 2, 3-Dioxygenase: Is it an Immune Suppressor?,” The Cancer Journal, 16 (4): 354-9.
Publisher – Google Scholar
Sugimoto, H., Oda, S., Otsuki, T., Hino, T., Yoshida, T. & Shiro, Y. (2006). “Crystal Structure of Human Indoleamine 2,3-Dioxygenase: Catalytic Mechanism of O2 Incorporation by a Heme-Containing Dioxygenase,” PNAS, 103(8):2611-6.
Publisher – Google Scholar
Taylor, M. W. & Feng, G. S. (1991). “Relationship between Interferon-Gamma, Indoleamine 2,3-Dioxygenase, and Tryptophan Catabolism,” The FASEB Journal, 5(11): 2516-22.
Publisher – Google Scholar
Terness, P., Bauer, T. M., Rose, L., Dufter, C., Watzlik, A., Simon, H. & Opelz, G. (2002). “Inhibition of Allogeneic T Cell Proliferation by Indoleamine 2,3-Dioxygenase-Expressing Dendritic Cells: Mediation of Suppression by Tryptophan Metabolites,” The Journal of Expiremental Medicine, 196: 447—57.
Publisher – Google Scholar
Thomas, S. R. & Stocker, R. (1999). “Redox Reactions Related to Indoleamine 2,3-Dioxygenase and Tryptophan Metabolism along the Kynurenine Pathway,” Redox Report, 4: 199—220.
Publisher – Google Scholar
Tone, S., Takikawa, O., Habara-Ohkubo, A., Kadoya, A., Yoshida, R. & Kido, R. (1990). “Primary Structure of Human Indoleamine 2,3-Dioxygenase Deduced from the Nucleotide Sequence of its cDNA,” Nucleic Acids Research, 18(2): 367.
Publisher – Google Scholar
Uyttenhove, C., Pilotte, L., Theate, I., Stroobant, V., Colau, D., Parmentier, N. et al. (2003). “Evidence for a Tumoral Immune Resistance Mechanism Based on Tryptophan Degradation by Indoleamine 2,3-Dioxygenase,” Nature Medicine , 9: 1269—74.
Publisher – Google Scholar
Vantyghem, M. C., Marcelli-Tourvielle, S., Pattou, F. & Noel, C. (2007). “Effects of Nonsteroid Immunosuppressive Drugs on Insulin Secretion in Transplantation,” Annales d’Endocrinologie (Paris), 68: 21—27.
Publisher – Google Scholar
Varga, J., Yufit, T., Hitraya, E. & Brown, R. R. (1996). “Control of Extracellular Matrix Degradataion by Interferon-Gamma. The Tryptophan Connection,” Advances in Experimental Medicine and Biology, 398: 143-8.
Publisher – Google Scholar
Vazquez, S., Parker, N. R., Sheil, M. & Truscott, R. J. W. (2004). “Protein-Bound Kynurenine Decreases with the Progression of Age-Related Nuclear Cataract,” Investigative Ophthalmology & Visual Science, 45: 879—83.
Publisher – Google Scholar
Werner-Felmayer, G., Werner, E. R., Fuchs, D., Hausen, A., Reibnegger, G. & Wachter, H. (1989). “Characteristics of Interferon Induced Tryptophan Metabolism in Human Cells in Vitro,” Biochimica et Biophysica Acta (BBA) – Molecular Cell Research, 1012: 140-47.
Publisher – Google Scholar
Wichers, M. C. & Maes, M. (2004). “The Role of Indoleamine 2,3-Dioxygenase (IDO) in the Pathophysiology of Interferon-α-Induced Depression,” Journal of Psychiatry and Neuroscience, 29:11—17.
Publisher – Google Scholar
Yoshida, R., Urade, Y., Nakata, K., Watanabe, Y. & Hayaishi, O. (1981). “Specific Induction of Indoleamine 2,3-Dioxygenase by Bacterial Lipopolysaccharide in the Mouse Lung,” Archives of Biochemistry and Biophysics, 212(2): 629-37.
Publisher – Google Scholar
Zahr, E., Molano, R. D., Pileggi, A., Ichii, H., Jose, S. S., Bocca, N., An, W., Gonzalez- Quintana, J., Fraker, C., Ricordi, C. & Inverardi, L. (2007). ‘Rapamycin Impairs in Vivo Proliferation of Islet β-Cells,’ Transplantation, 84: 1576—83.